Figures & data
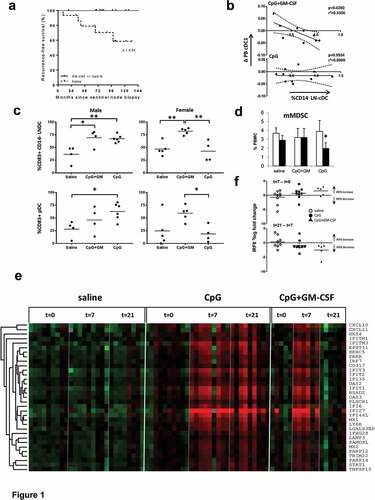
Figure 1. Added local and systemic effects of GM-CSF, co-delivered locally with CpG-B, in early-stage melanoma. Results shown are from patients receiving either a saline placebo (saline), or two administrations of CpG-B (CpG, 1 mg) or CpG combined with GM-CSF (CpG+GM, 1 mg + 100 µg) at day −7 and day −2 before sentinel lymph node biopsy (SNB), or GM-CSF alone (GM-CSF), 4 doses of 3µg/kg divided over the four days leading up to SNB. All were administered at the excision site of the primary tumor. (a) Recurrence-free survival of patients who were treated with GM-CSF with or without CpG-B (n = 14) versus patients that received saline (n = 15, p value is listed). (b) Correlation between changes in BDCA3/CD141+ peripheral blood cDC (cDC1) frequencies (between day −7 and 0) and CD1a−CD11chiCD14− cDC (CD14-LNDC) rates in the SLN of patients who received CpG+GM or CpG. (c) CD14− LNDC and pDC activation (by CD83) in men and women after local treatment with CpG or CpG+GM. (d) Pre- (day −7, open bars) and post-treatment (day 0, closed bars) frequencies of monocytic myeloid derived suppressor cells (mMDSC) in peripheral blood of patients receiving either saline, CpG, or CpG-B+ GM-CSF. (e) Transcriptional profiling reveals post-treatment induction of a type-I Interferon (IFN) response signature in peripheral blood mononuclear cells in patients receiving CpG or CpG+GM. (f) Changes in IRF8 transcript levels (relative to GAPDH) between t = 7 and t = 0 and t = 21 and t = 7 for saline (n = 9); CpG (n = 9); CpG+GM-CSF (n = 5). Statistical significance: * P < .05; ** P < .01; either by One-way ANOVA with Tukey post-hoc test or by paired two-sided student’s T test