Figures & data
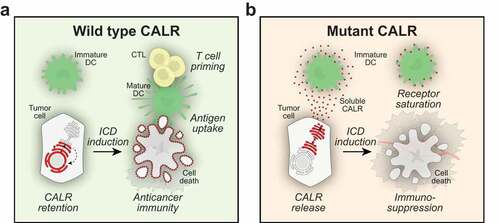
Figure 1. Immunosuppression by soluble calreticulin. Calreticulin (CALR) can be exposed on the cell surface of cells undergoing immunogenic cell death. Surface-exposed CALR then serves as an uptake signal for dendritic cells (DC), thereby facilitating the transfer of tumor-associated antigens to DC and ultimately the priming of cytotoxic T lymphocytes (CTL) (a). Mutations of the (CALR) gene occur in many forms of cancer and the loss of the KDEL retention signal can lead to the expression of mutant CALR that is secreted from the cells. Extracellular CALR binds to DC and inhibits phagocytosis, presumably by saturating a specific receptor for CALR. Through this mechanism, soluble CALR exerts immunosuppressive effects, hence subverting the effects of anticancer immunotherapy (b)