Figures & data
Figure 1. Stat3 silencing induces senescence in cancer cells addicted to Stat3 signaling pathway
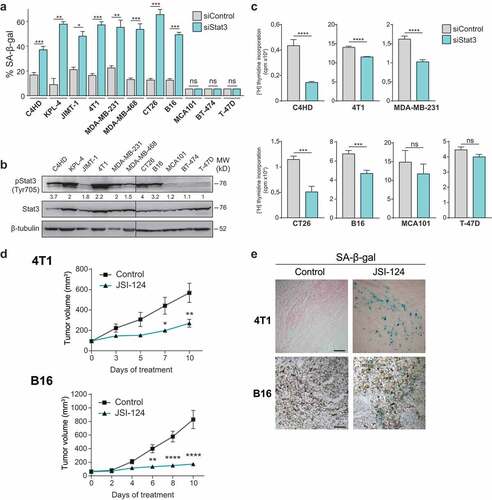
Figure 2. Antitumor activity of SASP from Stat3-silenced cancer cells
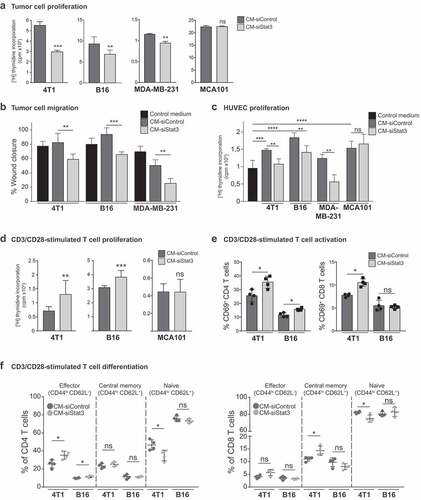
Figure 3. Immunotherapy of SASP from Stat3-silenced 4T1 cells with irradiated wild type cells promotes an antitumor immune response
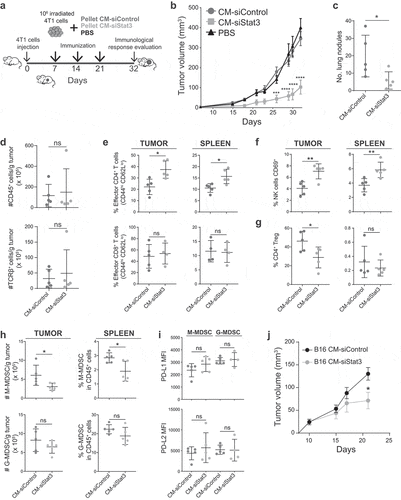
Figure 4. Immunotherapy with SASP from Stat3-silenced cancer cells acts in synergy with anti-PD-1 antibodies
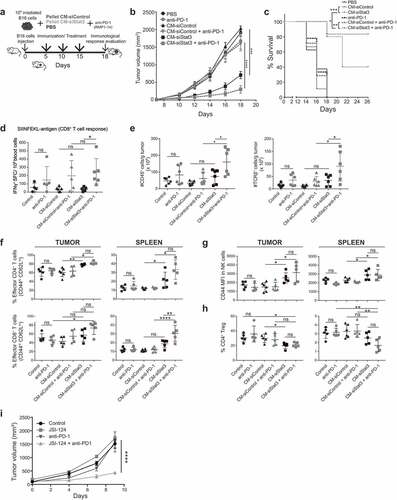
Figure 5. CCL2, CCL5, IL-15, and CXCL10 are mediators of the immune-stimulating activity of the SASP from Stat3-silenced cancer cells
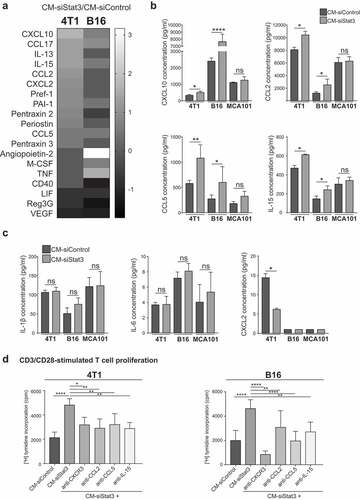
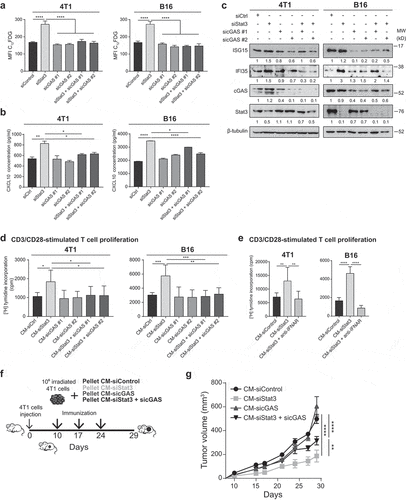
Figure 6. Senescence and type I IFN-related protein production induced by Stat3 silencing is dependent on cGAS