Figures & data
Figure 1. CD34+ HSPC from adult sources show slower in vitro maturation kinetics and less expansion compared to cord blood HSPC. (a) Culture protocol. Numbers indicate time (in days) of co-culture. Abbreviations: CB, cord blood; mPB, mobilized peripheral blood; BM, bone marrow; DP, double positive; SCF, stem cell factor; FLT3-L, FLT3 ligand; IL, interleukin; RV, retroviral. (b) Kinetics of expansion before transduction in OP9-DL1 co-cultures of HSPC from cord blood (n = 7), healthy donors (n = 12), patients in remission after chemotherapy (n = 15) and AML patients at diagnosis (n = 12). Mean ± s.d. is shown. T-cell committed progenitors in cord blood co-cultures were transduced at day 14, in co-cultures from adult HSPC at later timepoints (d19 or d24). (c) Relative cell numbers (i.e. cell numbers obtained when theoretically starting from a single CD34+ cell at day 0) at day 14 of co-cultures from cord blood (n = 7), healthy donor (n = 13), patient in remission after chemotherapy (n = 16) and AML patient at diagnosis (n = 13) HSPC. Values for individual samples and mean ± s.d. are shown. Mann–Whitney U test was used to assess statistical significance. P-value < 0.05 (*), P < 0.01 (**) and P < 0.001 (***)
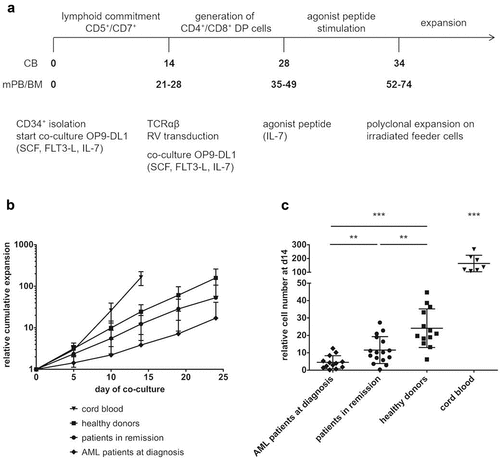
Figure 2. Presence of early T-progenitors at the start of co-cultures. Percentage of early T-progenitors (ETP, lin− CD34+ CD38+ CD7+) in CD34+ cells isolated at day 0 of co-cultures from cord blood (n = 7), healthy donors (n = 7), patients in remission (n = 5) and AML patients at diagnosis (n = 9). Individual samples, mean percentages per sample group and s.d. are shown. Mann–Whitney U test was used to assess statistical significance. P-value < 0.01 (**). Other differences were not significant
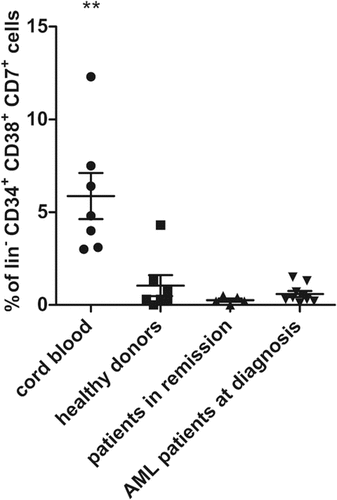
Figure 3. Multiple rounds of agonist peptide stimulation are needed to achieve the selection and maturation of HSPC from adult sources. Maturation of TCR-transduced cells after one or more rounds of agonist peptide stimulation. Contour plots show CD1a (x-axis) and CD27 (y-axis) expression before agonist stimulation and after 1, 2 or 3 rounds of agonist stimulation for TCR-transduced cells in co-cultures from cord blood (a), healthy donors (b), patients in remission after chemotherapy (c) and AML patients at diagnosis (d). Gating on eGFP+ TCR-transduced cells. Numbers indicate percentages of cells in each quadrant. A representative sample from each sample group is shown
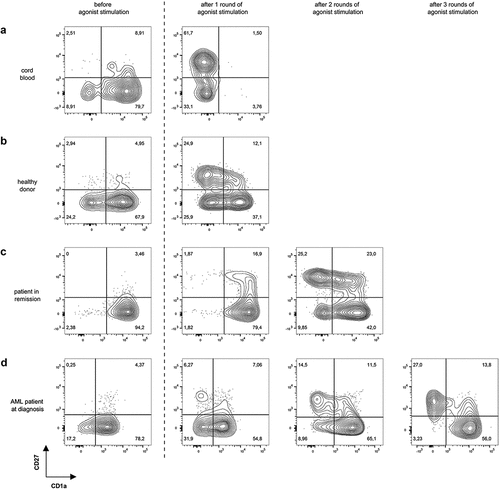
Figure 4. Phenotype of in vitro generated T-cells after agonist peptide stimulation and after polyclonal feeder expansion. (a) Percentage of cells with a TN (CD45RA+ CD62L+ CXCR3− CD95−), TSCM (CD45RA+ CD62L+ CXCR3+ CD95+), TCM (CD45RA− CD62L+ CXCR3+ CD95+), TEM (CD45RA− CD62L− CXCR3− CD95+) and TTE (CD45RA+ CD62L− CXCR3− CD95+) phenotype before agonist peptide stimulation, and after 1, 2 or 3 rounds of agonist peptide stimulation. (b) Percentage of cells with a TN, TSCM, TCM, TEM, and TTE phenotype after polyclonal feeder expansion. For (a) and (b) T-cells were generated from HSPC from healthy donors (n = 3), patients in remission (n = 6) and AML patients at diagnosis (n = 3). Gating on eGFP+ TCR-transduced cells. Mean and s.d. are shown. (c) Percentage of cells positive for PD-1 expression after polyclonal feeder expansion. T-cells were generated from HSPC from healthy donors (n = 8), patients in remission after chemotherapy (n = 9) and AML patients at diagnosis (n = 6). Gating on eGFP+ TCR-transduced cells. Individual samples and mean ± s.d. are shown. Mann–Whitney U test was used to assess statistical significance. P-value < 0.05 (*)
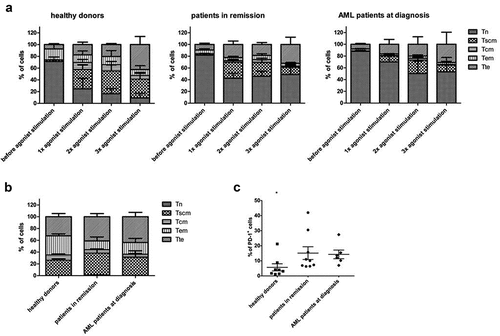
Figure 5. In vitro generated TA-specific T-cells specifically recognize and kill TA-expressing cell lines. (a) Intracellular staining of T-cells for interferon-gamma (IFNg) after co-culture with THP-1 (HLA-A2+ WT1+) or JY (HLA-A2+ WT1−) cells. Culture medium was used as a negative control, stimulation with aCD3/aCD28 as a positive control. Effector/target ratio 1/2. Gating on eGFP+ TCR-transduced cells. T-cells generated from HSPC from healthy donors (n = 4), patients in remission after chemotherapy (n = 5), AML patients at diagnosis (n = 4) and PBL (n = 6). (b) Percentage specific lysis determined via 4-h 51chromium release assay after co-culture of T-cells with T2 cells pulsed with relevant WT1 or irrelevant influenza (INF) peptide (10 µg/ml), HL-60-A2 (HLA-A2+ WT1+), THP-1, or JY cells. Effector/target ratio 10/1. T-cells generated from HSPC from healthy donors (n = 6), patients in remission after chemotherapy (n = 7), AML patients at diagnosis (n = 8) and PBL (n = 6). For (a) and (b) mean and s.d. are shown. Kruskal–Wallis test was used to determine statistical significance between different HSPC sample groups. Differences were not significant. Mann–Whitney U test was used to determine statistical significance for between-group comparisons for HSPC- and PBL-derived T-cells. P-value < 0.05 (*) and P < 0.01 (**)
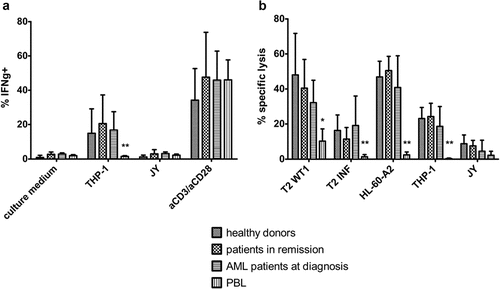
Figure 6. Cryopreservation has a negligible effect on maturation kinetics and the functionality of in vitro generated TA-specific T-cells. (a) Relative cell numbers at day 14 of co-cultures (day at which cord blood progenitors were transduced) from fresh and cryopreserved (frozen) HSPC from cord blood (n = 6), healthy donors (n = 6), patients in remission after chemotherapy (n = 7) and AML patients at diagnosis (n = 6). Individual fresh and paired frozen samples are shown. (b) Percentage specific lysis determined via 4-h 51chromium release assay after co-culture of T-cells with T2 cells pulsed with relevant WT1 or irrelevant influenza (INF) peptide (10 µg/ml), HL-60-A2 (HLA-A2+ WT1+), THP-1 (HLA-A2+ WT1+) or JY (HLA-A2+ WT1−) cells. Effector/target ratio 5/1. T-cells generated from fresh and cryopreserved (frozen) HSPC (n = 11). Results from different sample groups (healthy donors, patients in remission and AML patients at diagnosis) were pooled. Mean and s.d. are shown. Wilcoxon matched-pairs signed-rank test was used to assess statistical significance. Differences were not significant
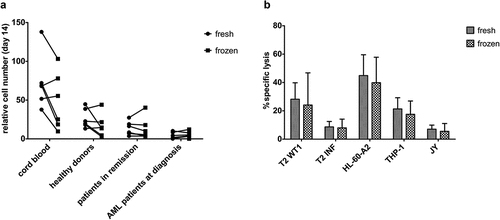
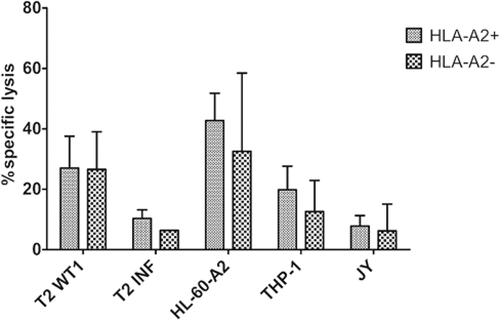
Figure 7. Functional TA-specific T-cells can also be generated from HSPC from HLA-A2 negative donors. Percentage specific lysis determined via 4-h 51chromium release assay after co-culture of T-cells with T2 cells pulsed with relevant WT1 or irrelevant influenza (INF) peptide (10 µg/ml), HL-60-A2 (HLA-A2+ WT1+), THP-1 (HLA-A2+ WT1+) or JY (HLA-A2+ WT1−) cells. Effector/target ratio 5/1. Mean and s.d. are shown. T-cells generated from HLA-A2+ (n = 5) and HLA-A2− (n = 4) HSPC. Results from different sample groups (healthy donors, patients in remission and AML patients at diagnosis) were pooled. Mann-Whitney U test was used to assess statistical significance. Differences were not significant