Figures & data
Figure 1. VPA delays the progression of EL4 and B16-F10 tumors
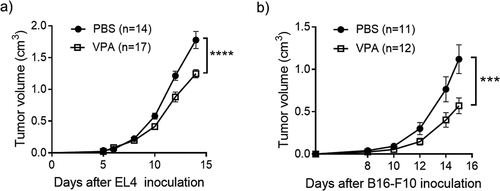
Figure 6. VPA enhances response to anti-PD-1 immunotherapy
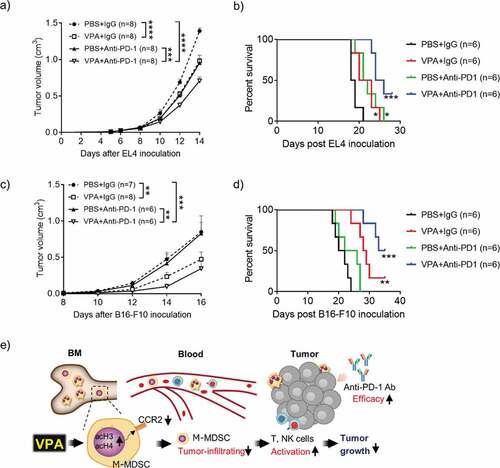
Figure 2. VPA reactivates tumor-infiltrating immune cells
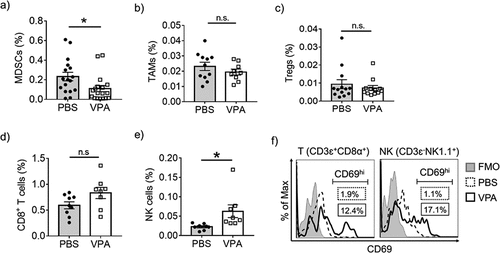
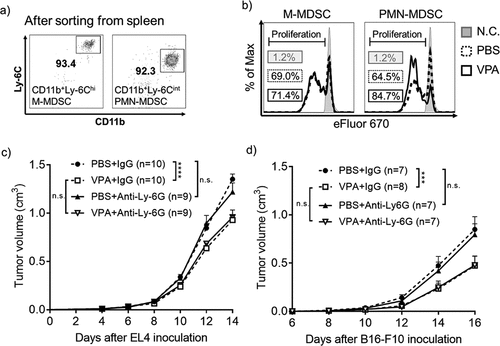
Figure 3. VPA impairs EL4 tumor progression via anti-tumor immune cells
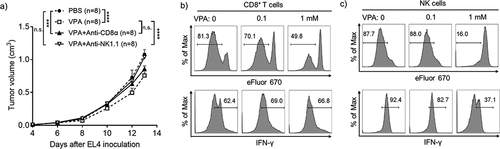
Figure 4. VPA reduces the infiltration of M-MDSCs in EL4 tumors
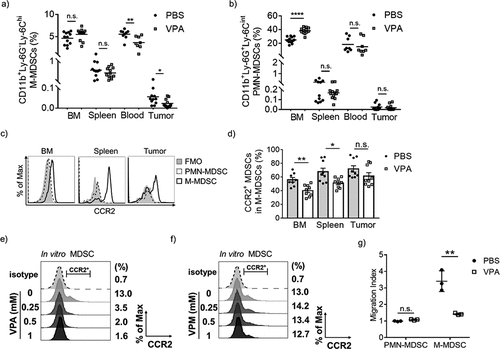
Figure 5. VPA attenuates the immunosuppressive activity of MDSCs in vivo.