Figures & data
Figure 1. Generation and characterization of anti-CD19 scFv fusion protein
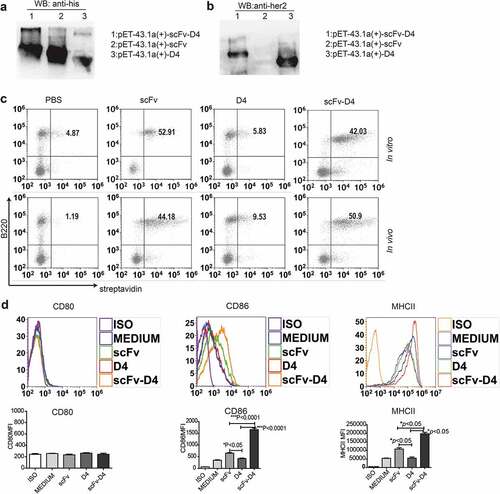
Figure 2. CD19-mediated Ag targeting of B cells induces antibody and T cell responses
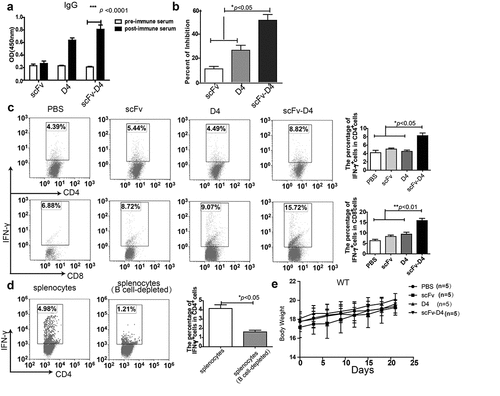
Figure 3. Targeting Ags to B cells induces anti-tumor activity in a 4T1/E2 breast cancer model
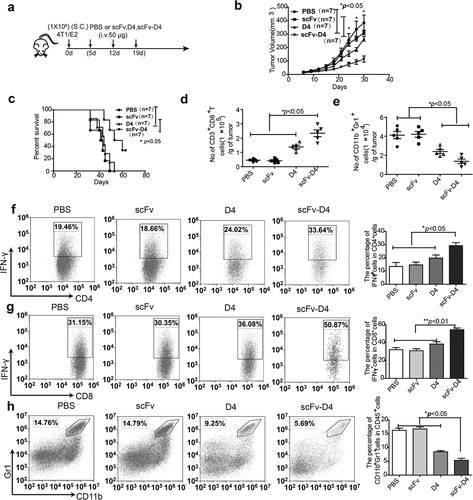
Figure 4. Targeting Ags to B cells promotes PD1 and PDL1 expression in the tumor microenvironment
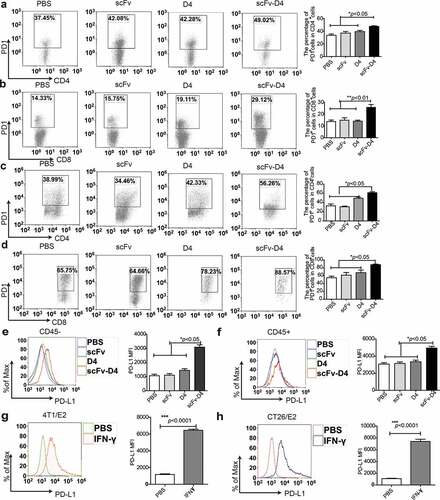
Figure 5. Combined treatment with scFv-D4 and anti-PD-1 induces immune cell infiltration and shows remarkable anti-tumor activity in a 4T1/E2 breast cancer model
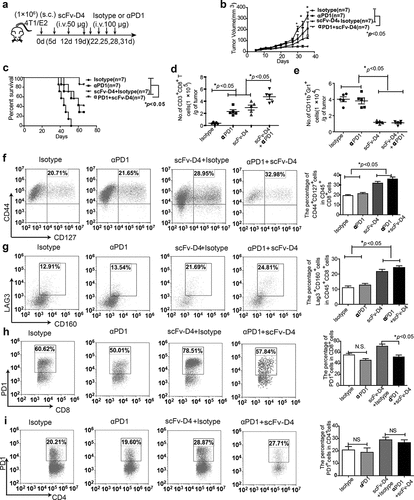
Figure 6. scFv-D4 and anti-PD1 combination therapy enhances T cell responses in a 4T1/E2 tumor model
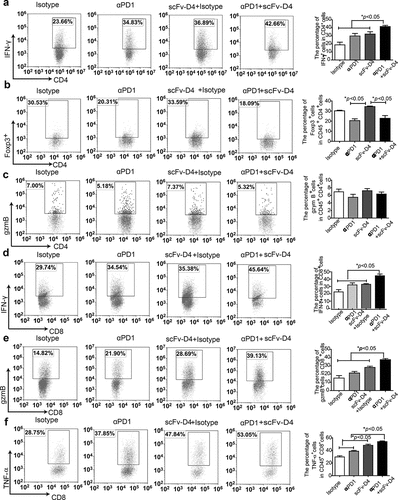
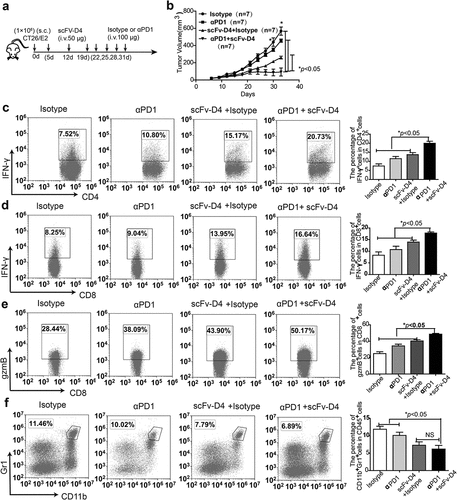
Figure 7. Treatment with fusion protein scFv–D4 and anti-PD1 induces significant anti-tumor effects in a CT26/E2 tumor model