Figures & data
Figure 1. Circulating neutrophils in kidney cancer patients display an immature-like phenotype. Blood leukocytes from 14 healthy donors (HD, open symbols) and 19 kidney cancer patients (closed symbols) were phenotyped by flow cytometry, and neutrophils were defined as CD45+, CD3−, CD66b+. (a) Neutrophil activation was measured by median fluorescence intensity (MFI) of surface CD14, CD16 and CD66b. (b) Primary granule release was quantified by surface CD63 expression and surface recapture of primary granule enzymes (arginase-1 and neutrophil elastase). (c) Immunomodulatory capacity of blood neutrophils was assessed by surface expression of PD-L1, HLA-DR and CD80. Differences between kidney cancer patients and healthy donors were determined using the Wilcoxon rank sum test
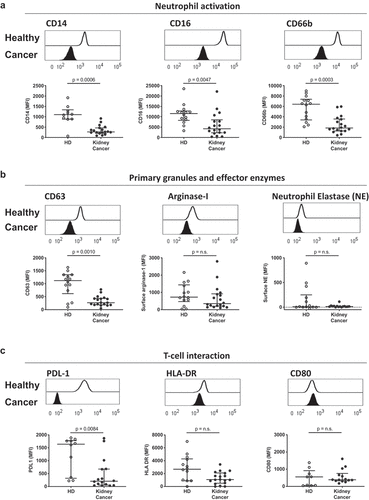
Figure 2. Circulating neutrophils from kidney cancer patients show enhanced metabolic activity. Circulating neutrophils from healthy donors (HD, open symbols) and kidney cancer patients (closed symbols) were plated on Seahorse culture plates in Seahorse media (± glucose and PMA, depending on the condition) and analyzed for maximal (a-b) and basal (c-d) extracellular acidification rate (ECAR), for spare respiratory capacity (e-f) and basal oxygen consumption rate (OCR) (g-h) as determinants of glycolysis and oxidative phosphorylation/NADPH activity, respectively. Glycolytic capacity was defined as the difference between maximal ECAR upon PMA activation and average ECAR upon glucose injection (glycolysis). Differences between kidney cancer patients and healthy donors were determined using the Wilcoxon rank sum test
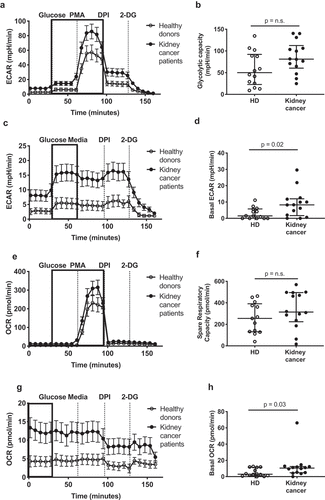
Figure 3. Tumor-associated neutrophils from kidney cancer patients display an immunosuppressive phenotype. (a) Representative flow cytometry plots of CD63 and arginase-1 expression on matched circulating (blood) and tumor neutrophils from kidney cancer patients. Data are shown for two kidney cancer patients. (b) Comparison of neutrophil activation and expression of immunomodulatory surface receptors. Differences between circulating and tumor-associated neutrophils within kidney cancer patients were determined using the Wilcoxon matched-pairs signed rank test
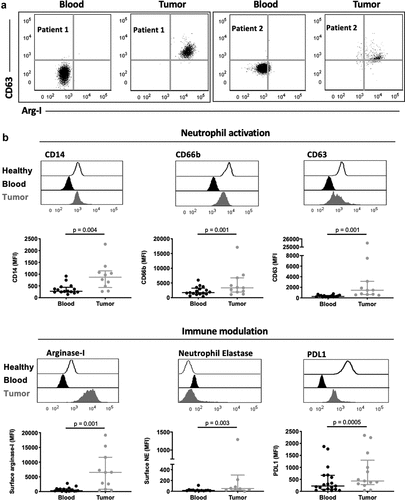
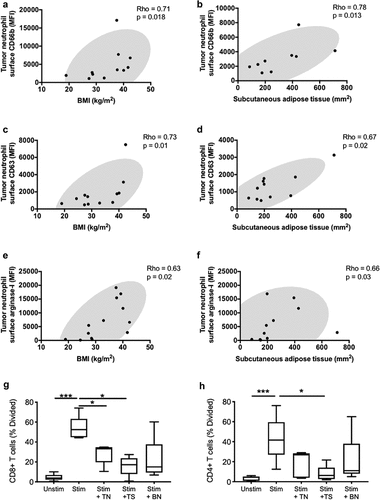
Figure 4. Systemic metabolic state correlates with degranulation by tumor-associated neutrophils. Correlations are shown between tumor-infiltrating neutrophil surface expression of granule release markers related to secondary granules (CD66b) (a-b), primary granules (CD63) (c-d) and the effector enzyme arginase-1 (e-f), and BMI or subcutaneous adipose tissue. BMI was calculated using the patients’ height and weight and subcutaneous adipose tissue area was calculated using an MRI dissection system as described in the methods. (g-h) Co-culture assay of T-cells with blood neutrophils (BN), tumor neutrophils (TN), and tumor supernatant (TS). Correlations were assessed using Spearman’s test, while comparison for the T cells assay was performed using the Friedman’s test with uncorrected Dunn’s test (* p < .05; *** p < .001)