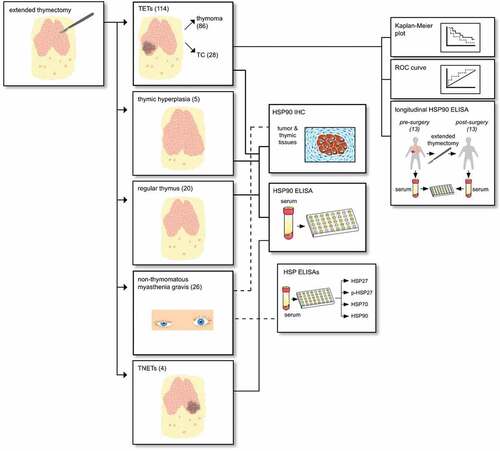
Figures & data
Table 1. HSP90α serum concentrations in TETs, basic demographic data and clinicopathologic characteristics. HSP90α serum concentrations in patients with TETs according to basic demographics, tumor staging systems (Masaoka-Koga and TNM 8th edition) and the WHO histological classification system.
Figure 2. Serum concentrations of HSP90α in patients with TETs. The differences in serum concentrations of healthy volunteers, patients with regular thymus as well as TETs are depicted (a). HSP90α serum concentrations in healthy volunteers or patients with regular thymus in comparison to patients with thymomas and TCs are shown (b). An analysis HSP90α serum concentrations of the particularly rare cases of TNETs (n = 4) is shown (c). Further, the analysis of HSP90α serum levels in patients according to Masaoka-Koga stages (d), and the 8th TNM edition (e) are displayed. The longitudinal analysis of HSP90α serum concentrations before and after extended thymectomy for TETs is depicted (f). The FFR survival analysis in thymomas separated according to high or low HSP90 serum level status is depicted (g). The CSS analysis in thymomas separated according to high or low HSP90α serum level status is shown (h).
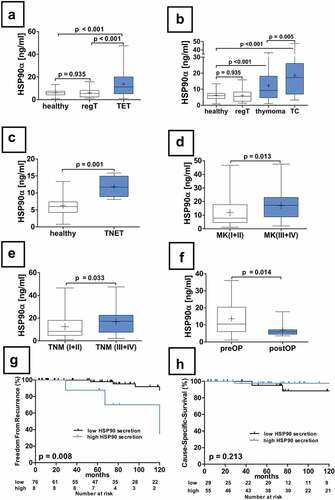
Figure 3. Serum concentrations of HSP90α in patients with non-thymomatous MG. The serum differences of HSP90α in patients with non-thymomatous MG, TET(MG+), TET (MG−) and healthy volunteers are shown (a). Further analysis of non-thymomatous MG patients according to the detection of antibodies to acetylcholine receptor antibodies (seropositive vs. seronegative is depicted (b). Moreover, the analysis of pHSP27 serum concentrations in patients with non-thymomatous MG receiving treatment with steroids is shown (c). The HSP90α serum concentration in patients improved after thymectomy and those who did not respond properly is depicted (d).
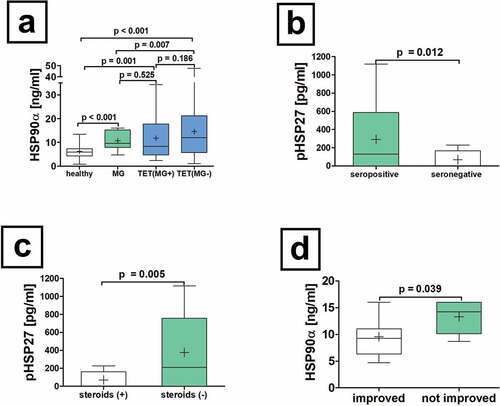
Table 2. Tumor expression of HSP90 in TET patients with or without MG. Immunohistochemical evaluation of tumor HSP90 expression in relation to WHO histological subtype classification is shown separately for TETs with and without MG. HSP90 expression was evident in nuclear and cytoplasmic compartments of tumor cells and non-neoplastic lymphatic cells.
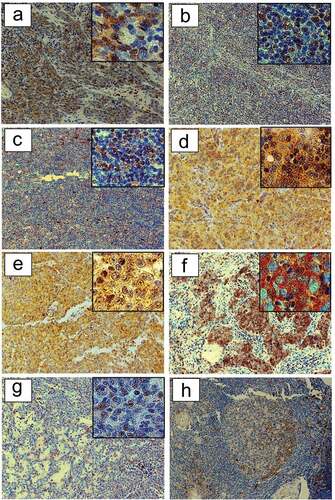
Figure 4. HSP90 expression in TETs. Immunohistochemical expression of HSP90 in WHO type A (a), AB (b), B1 (c), B2 (d), B3 (e), TC- Squamous Cell Carcinoma (f) and MNT (g) is shown (20X and 40X magnification). The representative sections (ac, eg) are from patients without MG, while section (d) is from a patient with paraneoplastic MG. Immunohistochemical staining of HSP90 in patients with non-thymomatous MG: a germinal center is depicted (H).