Figures & data
Figure 1. Therapeutic strategy involving microbial products to circumvent primary resistance to anticancer treatments.
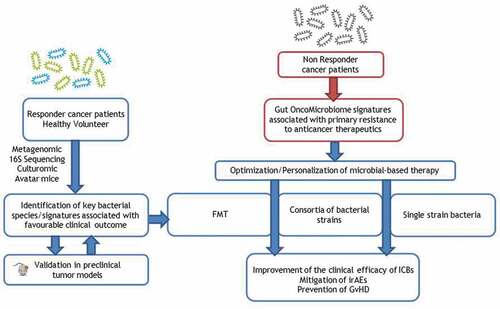
Table 1. Clinical trials employing microbial products for cancer therapy. AFMT: autologous fecal microbial transplantation; GvHD: graft-versus-host disease; ICBs: immune checkpoint blockers; irAE: immune-related adverse event; MDRB: multi-drug resistant bacteria; ORR: objective response rate; OS: overall survival; PFS: progression-free Survival; PSA: prostate-specific antigen; RRR: radiographic response rate; TKI: tyrosine kinase inhibitor.
