Figures & data
Figure 1. IL-33 causes eosinophil infiltration into tumors and reduced tumor growth in models of CRC.
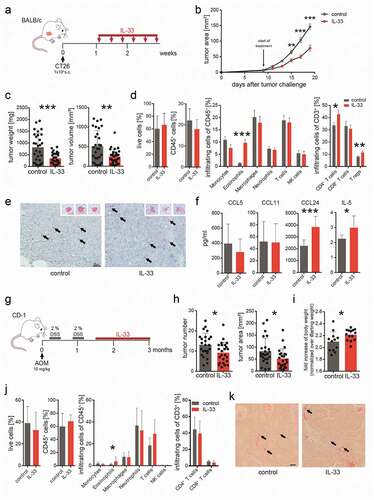
Figure 2. Expression of markers for activation, homing and degranulation.
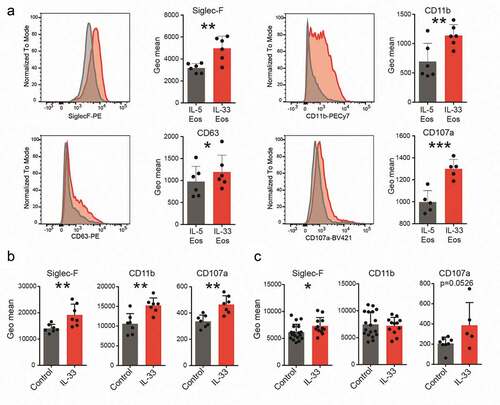
Figure 3. IL-33 dependent differences in migration and survival of eosinophils.
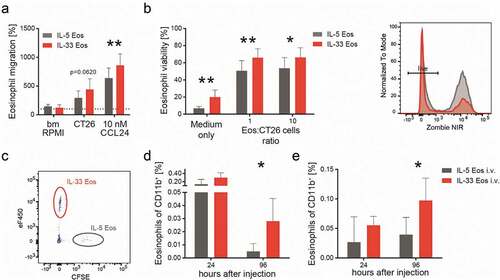
Figure 4. Eosinophils are necessary for a reduction in tumor growth by IL-33.
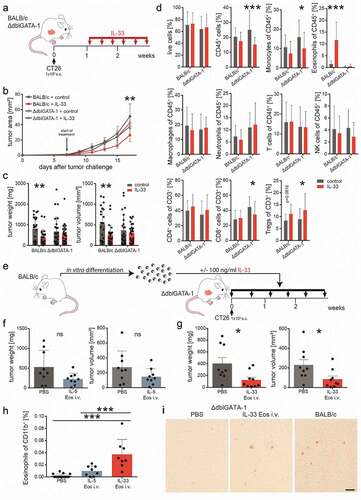
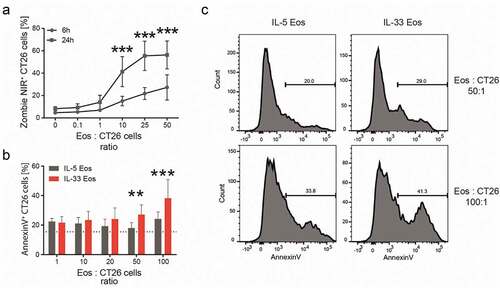
Figure 5. Cytotoxicity of eosinophils against CT26 cells in vitro.