Figures & data
Table 1. CALR as a biomarker in human cancer.
Table 2. Secreted CALR as a biomarker in myelofibrosis and solid tumors.
Figure 1. Surface-exposed calreticulin serves as an uptake signal for dendritic cells. Certain anticancer regimens induce T cell-dependent adaptive anticancer immunity via the initiation of immunogenic cell death (ICD). One of the apical hallmarks of ICD is a partial endoplasmic reticulum (ER) stress response that leads to the phosphorylation of eIF2α in the absence of other manifestations of the unfolded protein response. The resultant exposure of calreticulin (CALR) on the surface of dying cells facilitates their recognition by dendritic cells (DC) and thus enables tumor-associated antigen transfer culminating in adaptive anticancer immunity.
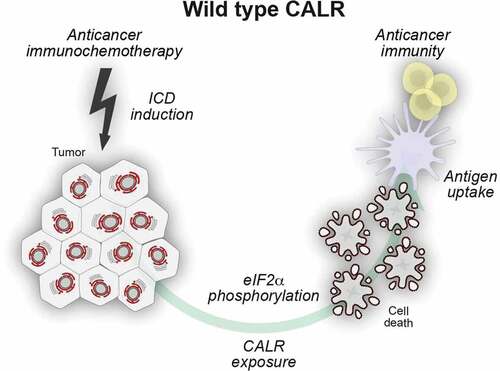
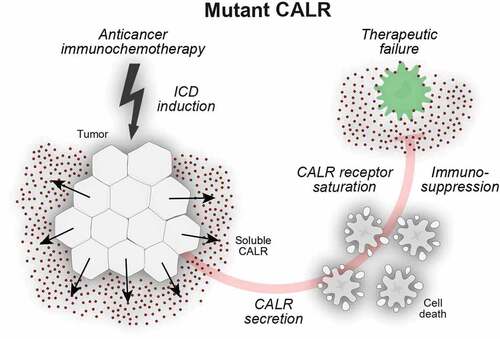
Figure 2. Secreted calreticulin negatively impacts on phagocytosis. Calreticulin (CALR) mutants that affect the C-terminal endoplasmic reticulum (ER)-retention signal KDEL can enter the canonical protein secretion pathway. Soluble CALR protein is secreted via Golgi-dependent exocytosis and ligates surface receptors on antigen presenting cells. In this setting, soluble CALR acts as a decoy that triggers receptor saturation and inhibits the dendritic cell (DC)-mediated phagocytosis of stressed and dying cancer cells, thus blunting adaptive immunity to tumor-associated antigens.