Figures & data
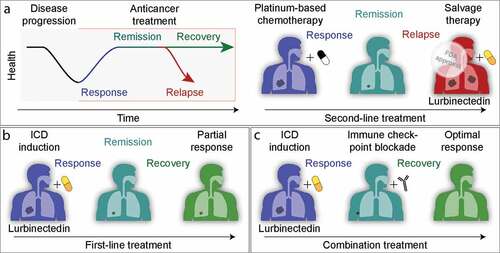
Figure 1. Clinical development of lurbinectedin for the treatment of small-cell lung carcinoma. a. Current FDA-approved use of lurbinectedin, as a salvage therapy after failure of platinum compound-based chemotherapy. b. Hypothetical use of lurbinectedin as a first-line treatment. c. Hypothetical combination of lurbinectedin used in first or second line with PD-1/PD-L1-blocking antibodies.