Figures & data
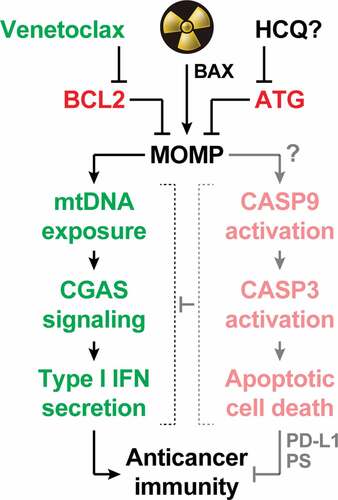
Figure 1. Mitochondrial apoptosis operates as a central rheostat to control the immunogenicity of irradiated cells. At least in some circumstances, radiation therapy (RT) can trigger abundant mitochondrial outer membrane permeabilization (MOMP) upon activation of BCL2 associated X, apoptosis regulator (BAX). In irradiated cells, MOMP is associated with the exposure of mitochondrial DNA (mtDNA) to the cytosol, which drives type I interferon (IFN) secretion via cyclic GMP-AMP synthase (CGAS). Conversely, it seems that RT-driven MOMP does not elicit potent caspase 9 (CASP9) and caspase 3 (CASP3) activation. This explains why type I IFN secretion and the consequent initiation of adaptive immune responses against irradiated cells has been documented in a variety of caspase-proficient models, despite the established capacity of active caspases to mediate immunosuppressive effects including CGAS cleavage, as well as CD274 (best known as PD-L1) and phosphatidylserine (PS) exposure. Both autophagy (ATG) and the anti-apoptotic protein BCL2 apoptosis regulator (BCL2) potently inhibit MOMP and its immunological consequences, delineating potential strategies for enhancing the immunogenicity (and hence the efficacy) of RT. While the safety and efficacy of ATG suppression with systemic hydroxychloroquine (HCQ) remain unclear, BCL2 inhibition with the clinically approved agent venetoclax stands out as a promising approach to clinical translation.