Figures & data
Table 1. Patient and tumor characteristics at baseline (ITT population)
Figure 1. CONSORT flow diagram of the LICC trial. * One patient in the tecemotide arm did not receive vaccination but received CP and was included in the ITT and safety population. In the tecemotide arm, one patient had one R2 resected metastasis besides R0 resected metastases and one patient had one RX metastasis besides R0 and R1 resected metastases. These patients were allocated to the “Non-R0” subgroup in the ITT population and were excluded from the PP population. A centralized radiologic review was conducted for 80 patients, which led to the exclusion of 11 patients (n = 7 tecemotide arm, n = 4 placebo arm) with residual disease at baseline from the per-protocol (PP) population. Abbreviations: ITT: intention-to-treat population, PP: per-protocol population
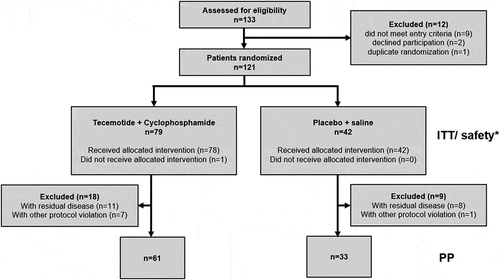
Figure 2. Kaplan-Meier analyses of the ITT population. A) Recurrence-free survival. B) Overall survival
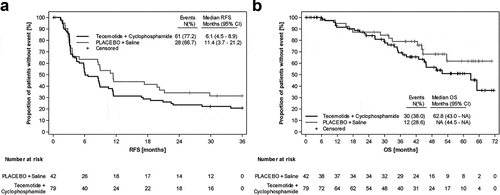
Figure 3. Kaplan-Meier analyses of the ITT population, according to MUC1 expression. A) RFS MUC1 low B) OS MUC1 low C) RFS MUC1 medium D) OS MUC1 medium E) RFS MUC1 high F) OS MUC1 high
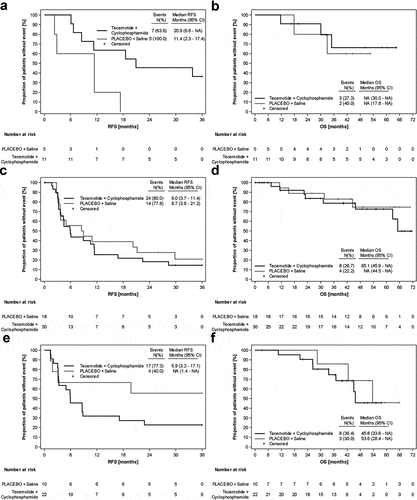
Figure 4. Forest Plots for the ITT population. A) Recurrence-free survival and B) Overall survival. P-values are for testing interaction of treatment with subgroup, thus describing difference in treatment effects across subgroups
Table 2. Overview of treatment-emergent adverse events (TEAEs) by treatment group – safety population
Table 3. Incidence and severity of treatment-emergent adverse events (TEAEs) by treatment group and by severity – safety population; Grade1/2 events are listed if ≥10% of patients were affected, grade 3/4 events are listed if at least two patients were affected
