Figures & data
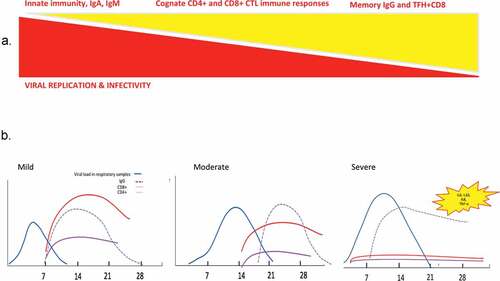
Figure 1. Natural history of COVID-19 infection, from incubation to critical disease.
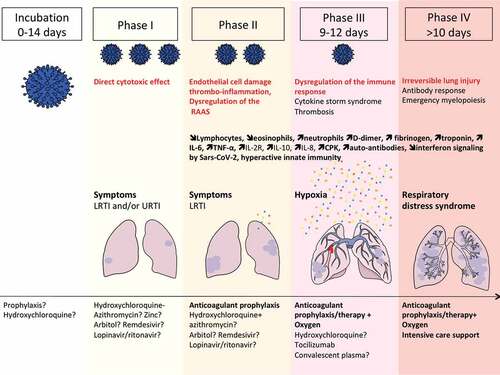
Figure 2. Extrapulmonary manifestations of COVID-19 identified in severe and critically ill patients (percentage in hospitalized patients).
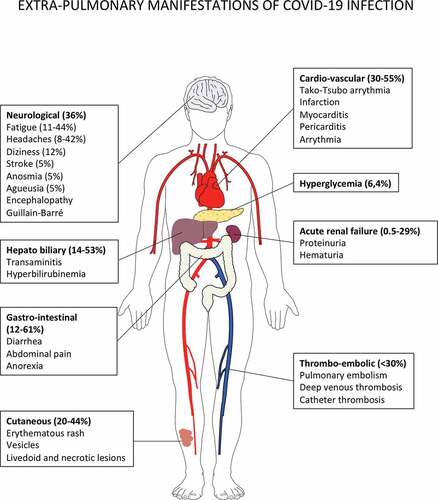
Figure 3. Putative immune scenarios associated with protective immune responses.
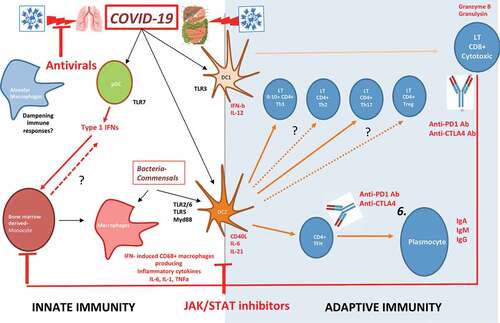
Figure 4. Bats are the natural reservoir of betacoronaviruses: immune specificities.
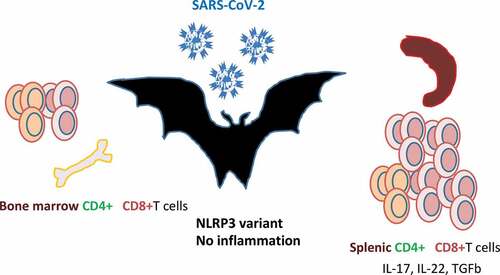
Figure 5. Race between viral replication and immune responses during viral infection with SARS-CoV viruses.