Figures & data
Figure 1. BsAb CD47xEGFR-IgG1 selectively and simultaneously binds to EGFR and CD47. Dose-dependent binding of CD47xEGFR-IgG1 to (a) CHO.EGFR or (b) CHO.CD47 cells vs. parental CHO cells. (c) Binding of CD47xEGFR-IgG1 or CD47xMock-IgG1 (1 µg/ml) to a panel of EGFRpos and EGFRneg CD47-expressing cell lines. (d) Binding of CD47xEGFR-IgG1 (1 µg/ml) to (GFP-labeled) CHO.CD47 cells and (DiD-labeled) parental A431 or A431.CD47KO cells in the presence or absence of excess CD47-blocking antibody B6H12 and/or EGFR-blocking antibody mAb 425 (both 10 µg/ml). The percentage of DiD/GFP double positive events upon incubation with indicated antibodies is shown. (e) Competitive binding assay in which APC-conjugated CD47 antibody (B6H12) competed with increasing doses (0.01–10 µg/ml) of bsAb CD47xEGFR-IgG1 (white squares), CD47xMock-IgG1 (black triangles) or MockxMock-IgG1 (white dots) for binding to A431 cells. (f) Competitive binding assay, as in E, in which A431 cells were pretreated with excess amounts of mAb 425 or isotype control IgG2a (both 10 µg/ml) prior to addition of CD47xEGFR-IgG1 (1 µg/ml), where indicated. (g) In vitro binding of CD47xEGFR-IgG1 and B6H12-hIgG1 to RBC present in whole blood. (h) Binding assay of CD47xEGFR-IgG1 (20 µg/ml) to DiO-labeled NCI-H292 cancer cells in the presence of increasing concentrations of whole blood. Of note, RBC in whole blood form an abundant “antigen-sink” for CD47-antibodies. All binding experiments were analyzed by flow cytometry. All graphs represent mean ± SD. Statistical analysis in F was performed using one-way ANOVA followed by a Tukey post-hoc test (*p < .05, **p < .01, ***p < .001, ****p < .0001, ns not significant)
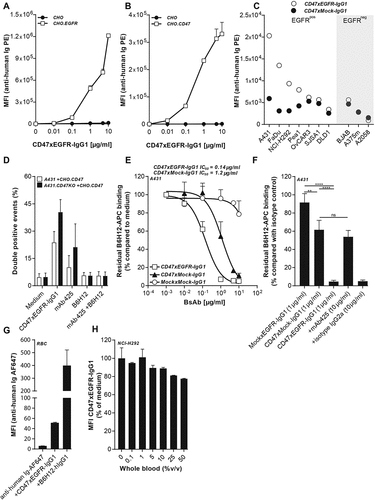
Figure 2. BsAb CD47xEGFR-IgG1 inhibits cancer cell proliferation and induces cointernalization of EGFR and CD47 from the cancer cell surface. (a) Cell proliferation of NCI-H292 cells was measured during 96 h of continuous treatment with bsAb CD47xEGFR-IgG1 or control antibodies (2 µg/ml) in an xCELLigence Real-Time Cell Proliferation assay. (b) FaDu cells were incubated with bsAb CD47xEGFR-IgG1 at 37°C and residual cell-surface presence of CD47 and EGFR were measured over time by flow cytometry and compared to medium control. Similar to B, residual cell-surface presence of CD47 (c) and EGFR (d) were measured on A431, NCI-H292, and FaDu cells after incubation with bsAb CD47xEGFR-IgG1 or control antibodies (1 µg/ml) at 37°C for 24 h. (e) Apoptosis of NCI-H292 cells upon internalization of EGFR and/or CD47 by indicated antibodies in the presence of Fab-ZAP, a chemical conjugate of goat anti-human monovalent antibody and saporin, was measured after 72 h. Apoptosis was measured with annexinV-staining using flow cytometry and expressed as percentage increase compared to medium control. (f) Representative confocal images of NCI-H292 cells stained with DAPI, EGFR antibody and CD47 antibody after 24 h internalization with bsAb CD47xEGFR-IgG1 or control antibodies (2 µg/ml). All graphs represent mean ± SD. Error bars in graph (a) and (b) indicate SD of duplicates. Statistical analysis in (c–e) was performed using One-way ANOVA followed by a Tukey post-hoc test (*p < .05, **p < .01, ***p < .001, ****p < .0001, ns not significant)
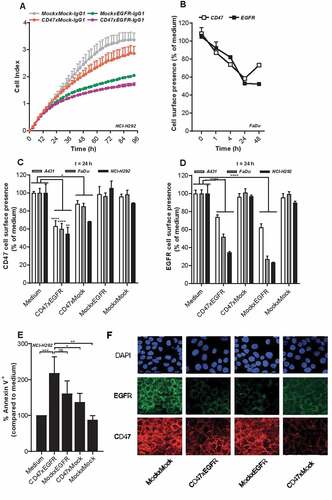
Figure 3. BsAb CD47xEGFR-IgG1 induces ADCC (a) ADCC reporter bioassay in which EGFRpos FaDu cells or EGFRneg A375m cells were coincubated with Jurkat. FcγRIIIa-NFAT-luc cells in the presence of bsAb CD47xEGFR-IgG1, CD47xEGFR-IgG2s, CD47xEGFR-IgG4, or CD47xMock-IgG1 (0.1 µg/ml). Luciferase activity (RLU) was measured after 6 h. (b) Evaluation of bsAb CD47xEGFR‐IgG1 and bsAb CD47xEGFR‐IgG2s for their respective capacity to engage ADCC activity of freshly isolated PBMCs from 5 healthy volunteers toward A431 cancer cells endogenously expressing EGFR and CD47. PBMCs were cocultured with DiD‐labeled A431 cancer cells at an E:T cell ratio of 5:1 for 24 h in the presence (or absence) of bsAb CD47xEGFR‐IgG1 or CD47xEGFRIgG2s (2 μg/ml each). Induction of apoptotic cancer cell death (annexin Vpos/PIpos) was assessed by flow cytometry. BsAb CD47xEGFR-IgG1 induces neutrophil-mediated trogocytosis and trogoptosis toward CD47posEGFRpos cancer cells. (c) FaDu or FaDu.CD47KO cells were DiD-labeled and incubated with neutrophils at an E:T cell ratio of 1:1 in the presence of anti-EpCAM-IgG1 (2 µg/ml), CTX or CD47xEGFR-IgG1 (both 1 µg/ml). Trogocytosis was quantified as the percentage of DiDpos neutrophils by flow cytometry after 2 h. (d) Similar as in (b), trogocytosis was measured after DiD-labeled FaDu cells were incubated with neutrophils (E:T cell ratio of 1:1) in the presence of increasing concentrations (0.01–1 µg/ml) of bsAb CD47xEGFR-IgG1, CD47xEGFR-IgG2s or CD47xEGFR-IgG4. (e) Next, neutrophil-mediated trogocytosis of a panel of EGFRpos and EGFRneg DiD-labeled CD47-expressing cell lines was assessed in the presence of bsAb CD47xEGFR-IgG1 or CD47xMock-IgG1 (both 1 µg/ml). (f) xCELLigence real-time cytotoxicity assay shows neutrophil-induced trogoptosis of FaDu cells opsonized with bsAb CD47xEGFR-IgG1, -IgG2s, or -IgG4. After adhering, FaDu cells were coincubated with IFNγ-stimulated neutrophils (E:T cell ratio of 10:1) in the presence of indicated antibodies (2 µg/ml). Cell index was normalized prior to addition of neutrophils and antibodies. All graphs represent mean ± SD. Graphs (c–e) represent data of three independent experiments with neutrophils from three different donors. Error bars in F indicate SD of quadruplicates. Statistical analysis in C was performed using one-way ANOVA followed by a Tukey post-hoc test (*p < .05, **p < .01, ***p < .001, ****p < .0001, ns not significant)
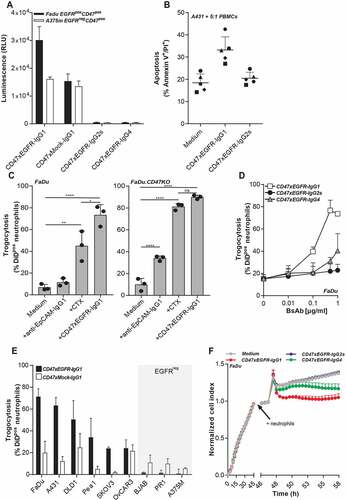
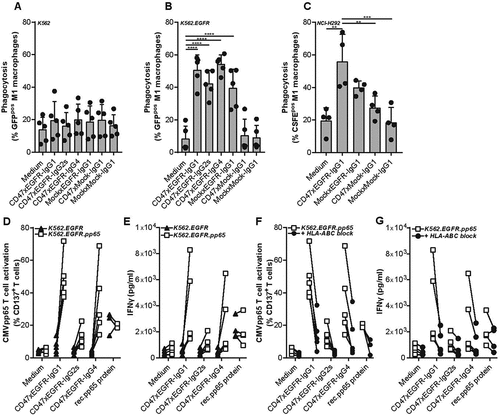
Figure 4. BsAb CD47xEGFR-IgG1-mediated phagocytosis of cancer cells by macrophages facilitates cross-presentation of peptides in MHC-I and subsequent activation of antigen-specific CD8+ T cells. Phagocytosis of GFP-labeled (a) K562, (b) K562.EGFR cells, or (c) CFSE-labeled NCI-H292 cells by M1 macrophages (E:T cell ratio of 1:2) in the presence of different isotypes of bsAb CD47xEGFR or control antibodies (1 µg/ml) after 3 h. (d) K562.EGFR cells or K562.EGFR.pp65 cells were coincubated with M1 macrophages from a CMV-seropositive donor (E:T cell ratio = 1:2) in the presence of CD47xEGFR-IgG1 or control antibodies (1 µg/ml) for 3 h. K562 cells and unbound antibody were washed away prior to addition of a priori expanded CMVpp65-specific autologous CD8+ T cells (E:T cell ratio of 1:1). After 24 h, the percentage of CD137pos T cells within CD8+ T cell population was measured by flow cytometry. (e) IFNγ levels in culture supernatant of D were measured by ELISA. (f) As in D, but HLA-ABC blocking antibody W6/32 (10 µg/ml) was added after addition of CMVpp65-specific autologous CD8+ T cells to block cross-presentation capacity of macrophages. (g) IFNγ levels in culture supernatant of F were measured by ELISA. Graphs (a–c) represent mean ± SD of independent experiments using at least three different donors. Statistical analysis in (a–c) were performed using One-way ANOVA followed by a Tukey post-hoc test. Graphs (d–g) represent independent experiments with macrophages from the same CMV-seropositive HLA-B7*07:02-matched donor (*p < .05, **p < .01, ***p < .001, ****p < .0001, ns not significant)