Figures & data
Figure 1. Coculture of PD-L1-expressing cells with PD-1-expressing cells induced lysosomal degradation of PD-1. (a) Total cell lysates of PC-9, PC9/PD-L1, Jurkat or Jurkat/PD-1 cells were collected and processed to western blot analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. (b) Confocal micrographs of PC-9 or PC9/PD-L1 cells probed with anti-PD-L1 (green) and DAPI (for nucleus, blue). Bar, 10 μm. (c) Flow cytometry analysis of PD-1 expression on cell surface of Jurkat or Jurkat/PD-1 cells after incubation with or without Alexa Fluor 488 anti-PD-1. NC, negative control. (d) PC9/PD-L1 and Jurkat/PD-1 cells were cocultured with each other for indicated time and then the whole cell lysates were processed for western blotting analysis using antibodies as indicated. The anti-GAPDH or anti-α-Tubulin antibody was used as a control for equal protein loading. The curves on the right indicated the quantitation of PD-1 or PD-L1 on the western blot relative to the control normalized by GAPDH or α-Tubulin. The relative protein levels were calculated by the gray scale scanning of AzureSpot software. (e) RT-qPCR analysis of PD-1 mRNA expression of Jurkat/PD-1 cells after coculturing with PC9/PD-L1 cells for 0 hr or 17 hr. ****p ≤ 0.001, Student’s t test. Error bars, mean±SD. (f) Jurkat/PD-1 and PC9/PD-L1 cells were treated with DMSO, 25 μM Chloroquine (CQ) or 50 nM bortezomib (BTZ) for 24 hr separately or in co-culture. The whole cell lysates were then collected and processed for western blotting analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. (g) Jurkat/PD-1 cells were cocultured with PC-9 or PC9/PD-L1 for 0 hr or 10 hr, and then the whole cell lysates were collected and processed for western blotting analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. (h) PC9/PD-L1 cells were pretreated with or without PD-L1 silencing RNA for 24 hr (PC9/PD-L1 (KD) or PC9/PD-L1), and then cocultured with Jurkat/PD-1 cells for another 0 hr or 17 hr. The whole cell lysates were processed for western blot using the antibodies as indicated. The anti-GAPDH or anti-α-Tubulin antibody was used as a control for equal protein loading. The histogram on the right quantified the level of PD-1 in each treatment which was normalized by GAPDH and quantified to the first treatment. ns, no significance, **p ≤ 0.005 and ****p ≤ 0.001, Student’s t test. Error bars, mean±SD.
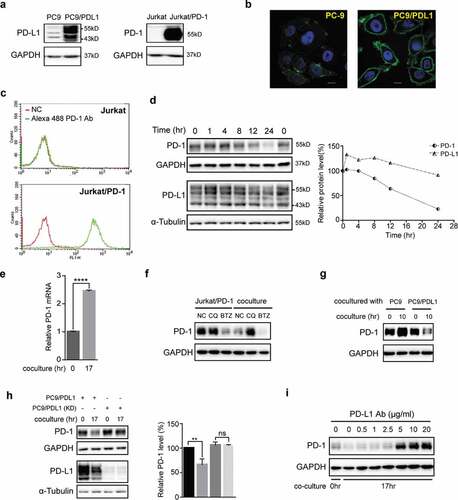
Figure 2. Small-molecule inhibitor BMS1166 blocked PD-L1/PD-1 interaction by targeting PD-L1. (a) Chemical structure of BMS1166. (b) Effects of anti-PD-L1 Ab or BMS1166 on PD-L1 and PD-1 in the coculture. PC9/PD-L1 cells were pretreated with indicated concentration of anti-PD-L1 Ab or BMS1166 for 1 hr, and then Jurkat/PD-1 cells were added in the culture for additional 0 hr or 17 hr. The whole cell lysates were collected and processed for western blotting analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. The histogram below indicated the quantitation of PD-1 on the western blot relative to the control normalized by GAPDH. (c) Effects of anti-PD-L1 Ab or BMS1166 on PD-L1 and PD-1 in separately cultured cells. PC9/PD-L1 or Jurkat/PD-1 cells were treated with DMSO (NC), 10 μg/ml anti-PD-L1 Ab (Ab) or 10 μM BMS1166 for 17 hr. Then the whole cell lysates were collected and processed for western blotting analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. (d) Effects of PD-L1 siRNA on PD-L1 protein. PC9/PD-L1 cells were transfected with or without PD-L1 siRNA (no. 1/2/3) for 24 hr. Total cell lysates were collected and processed for western blotting analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. (e) H1975 cells were treated with indicated concentration of BMS1166 or tunicamycin (TM) for 17 hr. Total cell lysates were collected and processed for western blotting analysis using antibodies as indicated. The anti-α-Tubulin antibody was used as a control for equal protein loading. (f) H1975 cells were incubated with indicated concentration of BMS1166 or TM in the presence or absence of 100 ng/ml IFN-γ for 17 hr. Total cell lysates were collected and processed for western blotting analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading.
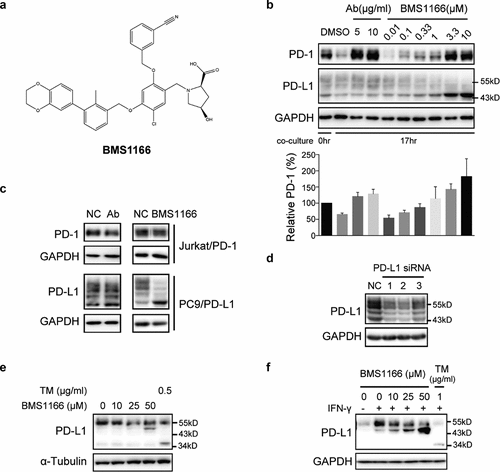
Figure 3. BMS1166 partially and specifically inhibited PD-L1 glycosylation. (a) PC9/PD-L1 cells were treated with DMSO, 10 μM BMS1166 or 1 μg/ml tunicamycin (TM) for 17 hr, and the whole cell lysates were collected and incubated with PNGase for 1 hr at 37°C, and then processed to western blot analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. (b) PC9/PD-L1 or Jurkat/PD-1 cells were treated with DMSO, 10 μM BMS1166 or indicated concentration of TM for 17 hr. Total cell lysates were collected and processed for western blot using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. (c) 4T1 cells were transfected with mPD-L1 plasmids for 24 hr and then incubated with DMSO (NC), 10 μM BMS1166 or 1 μg/ml TM overnight. Total cell lysates were collected and processed for western blot using antibodies as indicated. Anti-α-Tubulin antibody was used as a control for equal protein loading.
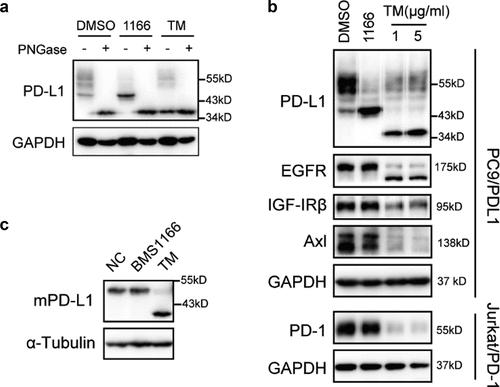
Figure 4. BMS1166 inhibited the glycosylation of PD-L1 variants and their interactions with PD-1. (a) Schematic diagram of PD-L1 mutants used in the study. Every red “×” indicates that the asparagine was substituted to glutamine. (b) and (c) 293 cells were transfected with indicated plasmid for 24 hr. They were then cultured alone (0 hr) or cocultured with Jurkat/PD-1 cells for 17 hr (17 hr) in (b). PD-L1 WT or mutants expressing 293 cells were incubated with or without 10 μM BMS1166 for 17 hr when cocultured with Jurkat/PD-1 cells in (c). “PD-L1/TM”, 293 cells were treated with 5 μg/ml TM overnight after transfection, which in order to compare the total expression level of non-glycosylated PD-L1. Whole cell lysates were processed for western blotting analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. The histogram below indicated the quantitation of PD-1 relative to the control normalized by GAPDH. *p ≤ 0.05, **p ≤ 0.005 and ***p < .001. ns, no significance. Student’s t test. Error bars, mean±SD.
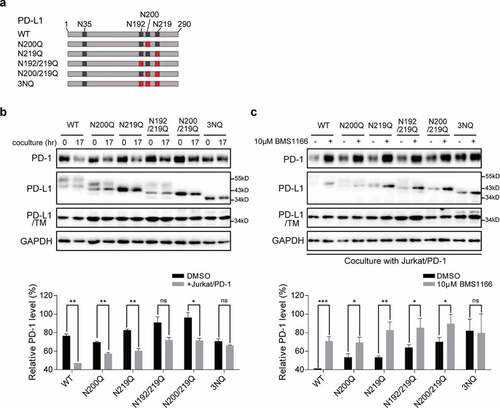
Figure 5. BMS1166 retained PD-L1 in ER and prevented its further glycosylation without inducing ER stress. (a) PC9/PD-L1 cells were treated with DMSO, 10 μM BMS1166 or 1 μg/ml TM for 17 hr, and the whole cell lysates were collected as “input”, and part of the lysates were incubated with agarose bound with Con A overnight as “Agarose bound Con A”. All the samples were processed to western blot analysis using antibodies as indicated. (b) PC9/PD-L1 cells were treated with DMSO, 10 μM BMS1166 or 1 μg/ml TM for 17 hr, and the whole cell lysates were collected and incubated with or without Endo H enzyme for 1 hr at 37°C. All the samples were processed to western blot analysis using antibodies as indicated. The anti-GAPDH antibody was used as a control for equal protein loading. (c) and (d) PC-9 cells were transfected with PD-L1-GFP plasmids for 24 hr and then treated with DMSO or 10 μM BMS1166 for 17 hr. Fixed samples were stained with ER-tracker red (1:1000) (c) or anti-GM130 (d) and 0.1 μg/ml DAPI, and then were visualized by confocal microscopy. White arrows point to co-localization of PD-L1-GFP and ER-tracker. Bar, 10 μm. (e) PC-9 or PC9/PD-L1 cells were incubated with DMSO (NC), BMS1166 or 1 μg/ml TM for 17 hr. Whole cell lysates were processed for western blotting analysis using antibodies as indicated. Anti-β-actin antibody was used as a control for equal protein loading. (f) PC-9 or PC9/PD-L1 cells were incubated with indicated concentrations of BMS1166 for 48 hr. Cell viability was assessed by MTT assay.
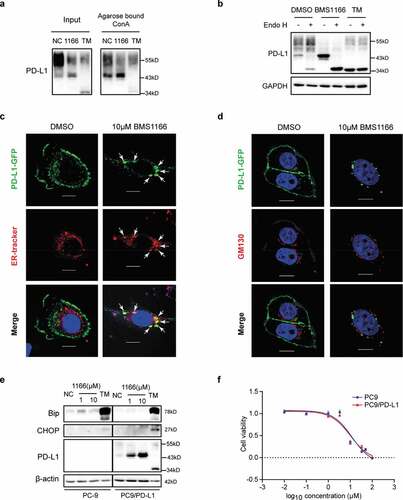
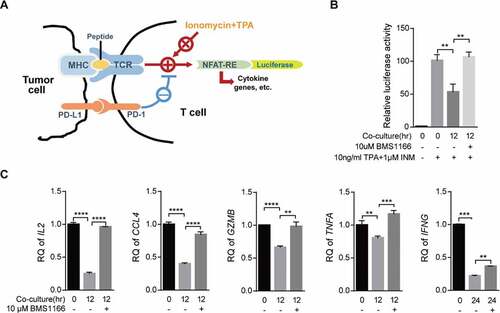
Figure 6. BMS1166 relieved immune suppression and activated T cell. (a) Schematic diagram of luciferase reporter gene assay. NFAT-RE, NFAT response elements. (b) and (c) PC9/PD-L1 cells were incubated with DMSO or 10 μM BMS1166 for 17 hr, and then cocultured with NFAT-luc-expressing Jurkat/PD-1 cells (b) or Jurkat/PD-1 cells (c) for 12 hr by concomitant treatment of 1 μM INM plus 10 ng/ml TPA. Cell lysates were collected to react with luciferase substrates (b) or analyzed by RT-qPCR. **, P < .01; ***, P < .001; ****, P < .0001, Student’s t test. Error bars, mean ± SD.
Supplemental Material
Download ()Data availability statement
All data generated or analyzed during this study are included in this published article.
