Figures & data
Figure 1. Dose, route, and timing of treatment administration by study arm. a LV305 is a NY-ESO-1 expressing, dendritic-cell tropic lentiviral vector. b G305 is recombinant NY-ESO-1 protein formulated in an oil-in-water stable emulsion with the synthetic TLR4 GLA. G305 dose for all study arms consisted of 250 μg NY-ESO-1 protein mixed with 5-μg GLA-SE. Patients were also given a boosting dose of G305 at each follow-up visit during the first year. c mCPA was only administered to patients in Arm C. It was dosed at 100 mg PO once daily for 7 days, then was not given for the next 7 days, in cycles that repeated until day 97. Patients were given a 1-week supply at each visit.d IT GLA-SE (5 µg/dose) was only administered to patients in Arm D and could have been injected into accessible primary tumors or distant metastases. If no accessible tumor was present at weeks 10, 11, 13, or 14, GLA-SE was not administered. Abbreviations: GLA-SE = glucopyranosyl lipid A-stable emulsion; ID = intradermal; IM = intramuscular; mCPA = metronomic cyclophosphamide; PO = oral; SC = subcutaneous; IT = intratumoral; μg = microgram; vg = viral genomes
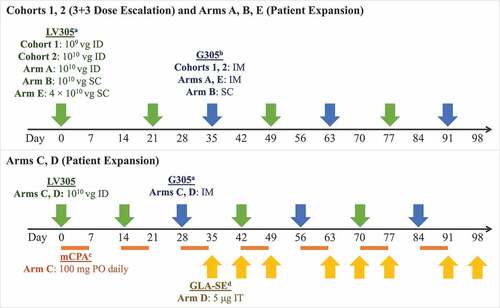
Table 1. Eligible tumor types and rationale for each study arm
Table 2. Patient demographics and baseline characteristics
Figure 2. Summary of adverse events by study arm. Three patients experienced dose-limiting toxicities, but there were no AEs or safety concerns reported with these dose-limiting toxicities. Two patients experienced treatment-related serious AEs in Arm A (prostatic pain in a patient with metastatic synovial sarcoma, and pneumonitis in a patient with non-small cell lung carcinoma who had a previous history of pneumonitis); no other patients experienced serious AEs considered related to treatment. One patient in Arm A experienced an AE of acute respiratory failure not considered related to treatment that led to death. Abbreviation: AE = adverse event
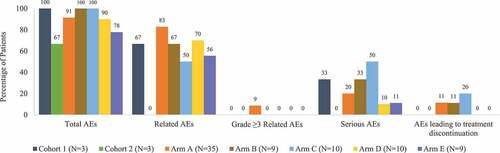
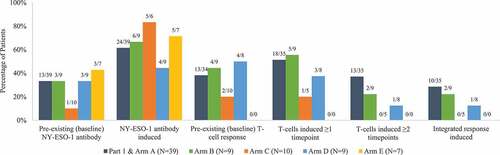
Figure 4. Immune response frequencies by study arm. Complete biomarker data were not available for all patients. The Ns for each study arm denote the total number of patients with biomarker data in that arm. Numerators and denominators are shown above each bar. Integrated response was defined as positive if both NY-ESO-1 antibody and T-cells (CD4 and CD8) were positive. T-cell analysis was not performed for patients in Arm E due to early study termination