Figures & data
Table 1. Demographics and baseline characteristics
Table 2. TRAEs of any grade occurring in ≥10% of patients
Table 3. Immune-related TRAEs
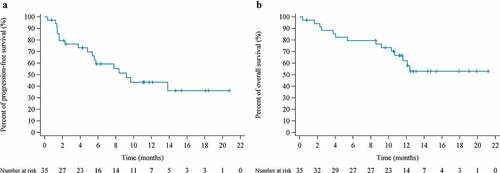
Table 4. Summary of response and survival data (FAS population)
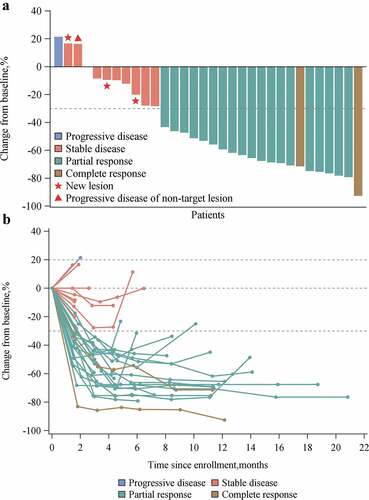
Figure 1. Overall tumor responses of HX008 with oxaliplatin plus capecitabine as assessed by site investigators in patients with ≥ 1 assessable postbaseline image assessment (N = 32). (A) Best change from baseline in the size of target tumor lesion. Color code defines the best of response of target tumor lesion. (B) Percent change in the size of target tumor lesion from baseline in each patient