Figures & data
Figure 1. B cell frequency, differentiation, phenotype and functionality in peripheral blood of melanoma patients. (a). B cells were identified as CD19+ and subtyped for naïve (IgD+CD27−), unswitched memory (IgD+ CD27+) and switched memory (IgD− CD27+) B cells. (b). B cell frequencies in blood as measured by flow cytometry from healthy donors (n = 34) and patients (n = 56), patients were further subdivided based on stage, including stage I/II (n = 8), III (n = 21) and IV (n = 27). (c). B cell differentiation as measured by flow cytometry and represented as frequency of B cells shown as naïve, switched and unswitched memory cells. Frequencies were analyzed from healthy donors (n = 34) and patients (n = 56), patients were further subdivided based on stage, including stage I/II (n = 8), III (n = 21) and IV (n = 27). (d). B cell phenotypes and function, assessed by flow cytometry, showing IL-6 R expressing B cells and analyzed from healthy donors (n = 21) and patients (n = 45), with stage I/II (n = 8), III (n = 13) and IV (n = 24). Cytokine production was assessed after stimulation with CpG (14 hours) and supplementary addition of PMA and ionomycin for 4 hours by flow cytometry in samples from healthy donors (n = 21) and patients (n = 45), with stage I/II (n = 8), III (n = 13) and IV (n = 24). For IL-10 the sample numbers were different: healthy donors (n = 33) and patients (n = 49), with stage I/II (n = 8), III (n = 15) and IV (n = 26). * p < .05, ** p < .01, *** p < .001 as determined by Kurskal-Wallis test
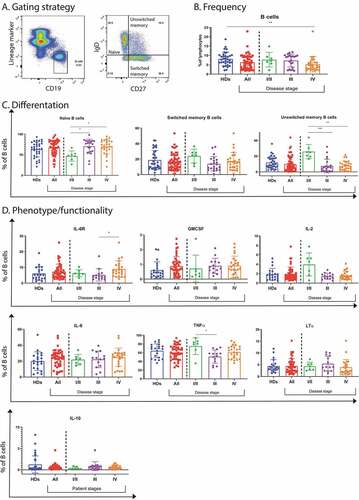
Figure 2. Circulating B cell frequency, differentiation, phenotype and response to anti-CTLA4 therapy in melanoma patients. (a). B cell frequency and differentiation as measured by flow cytometry including naïve, unswitched and switched memory B cells in healthy donors (n = 34) and patients (n = 21). (b). Phenotypical markers as assessed by flow cytometry including Fas receptor (CD95) and IL-6 receptor from healthy donors (n = 21) and patients (n = 22) as well as CXCR3 from healthy donors (n = 17) and patients (n = 14). (c). IL-6 receptor and cytokine expressing B cells in patients. They were defined as responders (R) (n = 8) when they had complete response, dissociated disease [at the same time presence of progression of some lesion(s) and of regression of other lesion(s)] or partial response. Non-responsive patients (NR) (n = 14) were defined when they had progressive disease. (d). Kaplan-Meier curves of overall survival of patients with lower (blue) or higher (red) frequencies of TNFα+ or IL-6+ B cells than the median. * p < .05, ** p < .01, *** p < .001 as determined by Mann-Whitney test
![Figure 2. Circulating B cell frequency, differentiation, phenotype and response to anti-CTLA4 therapy in melanoma patients. (a). B cell frequency and differentiation as measured by flow cytometry including naïve, unswitched and switched memory B cells in healthy donors (n = 34) and patients (n = 21). (b). Phenotypical markers as assessed by flow cytometry including Fas receptor (CD95) and IL-6 receptor from healthy donors (n = 21) and patients (n = 22) as well as CXCR3 from healthy donors (n = 17) and patients (n = 14). (c). IL-6 receptor and cytokine expressing B cells in patients. They were defined as responders (R) (n = 8) when they had complete response, dissociated disease [at the same time presence of progression of some lesion(s) and of regression of other lesion(s)] or partial response. Non-responsive patients (NR) (n = 14) were defined when they had progressive disease. (d). Kaplan-Meier curves of overall survival of patients with lower (blue) or higher (red) frequencies of TNFα+ or IL-6+ B cells than the median. * p < .05, ** p < .01, *** p < .001 as determined by Mann-Whitney test](/cms/asset/60a09e44-222a-4d01-8228-6ca9c7ae586c/koni_a_1873585_f0002_oc.jpg)
Figure 3. RNA sequencing analysis of B cells sorted from the TME, patient’s blood and healthy donor’s blood. (a). PCA analysis of B cells from the TME (red) and from the patient’s blood (black). (b). Volcano plot representation of the differential gene expression in B cells from the TME to B cells from patient’s blood. Adjusted p-value cutoff is 0.05 and log2(fold change) of −1 and 1. Hits passing these thresholds are represented in blue. (c). Bar graph representation of statistically significant enriched gene sets in B cells from the tumor compared to B cells from the patient’s blood. (d). PCA analysis of B cells from patient’s blood (red) and from healthy donor blood (black). (e). Volcano plot representation of the differential gene expression from B cells from patient’s blood to B cells from healthy donor blood. Adjusted p-value cutoff is 0.05 and log2(fold change) of −1 and 1. Hits passing these thresholds are represented in blue. (f). Bar graph representation of statistically significantly enriched gene sets in B cells from patient’s blood to B cells from healthy donor blood. (g). Venn diagram of up-and downregulated genes in B cells from patient’s blood compared to B cells from healthy donor’s blood as well as B cells from the tumor to B cells from the patient’s blood. Genes were considered significantly upregulated if the adjusted p-value<0.05 and log2(fold change)>1. Genes were considered significantly downregulated if the adjusted p-value<0.05 and log2(fold change)<-1. The total number of differently expressed genes is also indicated. H. Lists of up- and downregulated genes shown in Figure 3 G
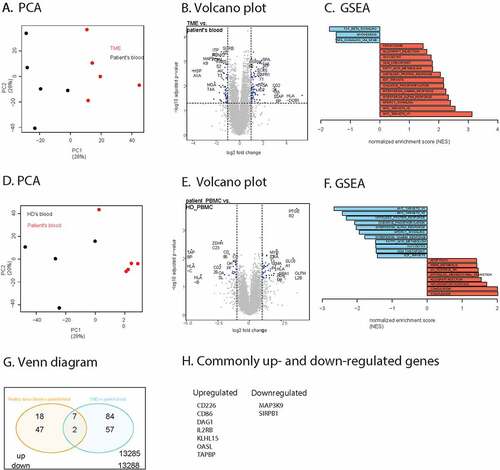
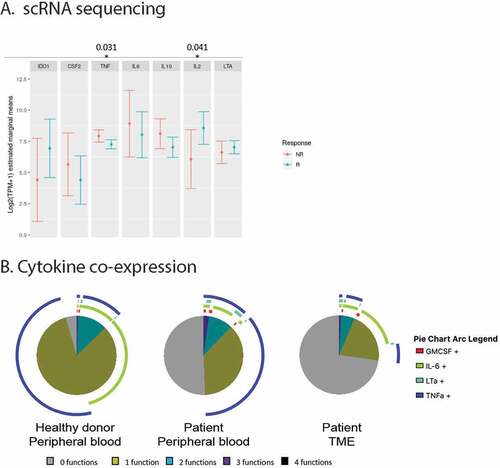
Figure 4. A. Distribution of mean gene expression in 1,379 single B cells, by response status (444 cells come from non-responders (NR) and 935 come from responders (R)). Mean expression values were estimated after fitting a zero inflated generalized linear mixed model to the expression profiles of B cells, setting response status as fixed effects and patient as a random effect. The bars represent the confidence intervals of the estimated means. B. Co-expression of cytokines as produced by B cells from respectively the peripheral blood of healthy donors (n = 3) or patients (n = 14), or the TME (n = 10)