Figures & data
Figure 1. KLRG1 knockout mice display fewer B16-E-cadherin tumors in the lungs
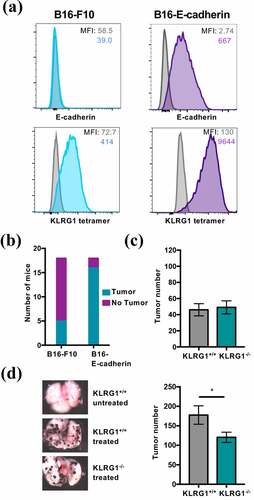
Figure 2. Combination KLRG1 and PD-1 therapy synergizes to decrease B16-E-cadherin tumor burden
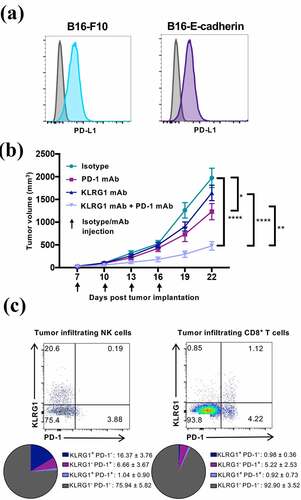
Figure 3. Combination therapy increases NK cell and CD8+ T cell frequency in the tumor microenvironment
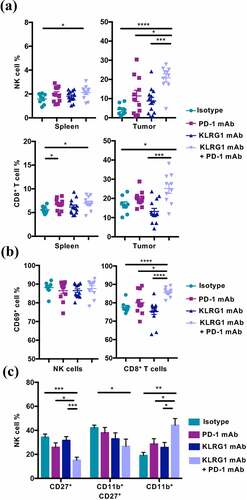
Figure 4. Combination KLRG1 and PD-1 therapy synergizes to decrease B16-F10 tumor growth
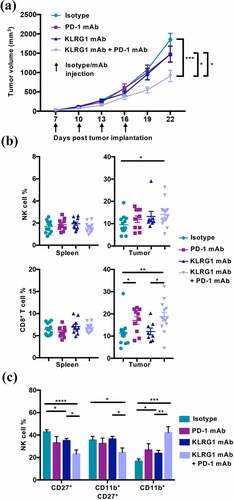
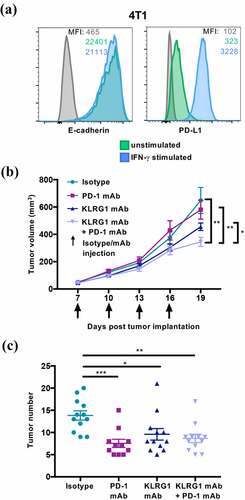
Figure 5. KLRG1 therapy alone decreases 4T1 tumor burden in the lungs while double blockade therapy decreases subcutaneous 4T1 tumor burden