Figures & data
Figure 1. IBI323 binds to human PD-L1 and LAG-3. (a) Schematic structure of IBI110, Bi127 and IBI323. (b) Binding affinity and kinetics of IBI323 and its parental Fc-fused PD-L1 single-domain antibodies to PD-L1 determined by surface plasma resonance. (c) Binding affinity and kinetics of IBI323 and its parental LAG-3 mAb to LAG-3 determined by surface plasma resonance. (d) Simultaneous binding of IBI323 to human PD-L1 and LAG-3 measured by biolayer interferometry. (e) Binding of IBI323 and its parental antibodies to PD-L1-expressing CHO-S cells. (f) Binding of IBI323 and its parental antibodies to LAG-3-expressing 293-F cells. Cells were incubated with serially diluted IBI323, IBI110, Bi-127 or IgG1 antibody, followed by a PE-conjugated anti-human IgG. MFI was determined by flow cytometry. (g) Flow cytometry analysis of PD-L1 and LAG-3 expression on activated CD4+ T cells. (h) Flow cytometry analysis of PD-L1 and LAG-3 co-expression in activated CD4+ T cells. (i) Primary cell-based binding assay for IBI323, its parental antibodies, and human IgG using activated human CD4+ T cells and anti-human Fc-PE secondary antibody. Data are representative of three independent experiments or three donors
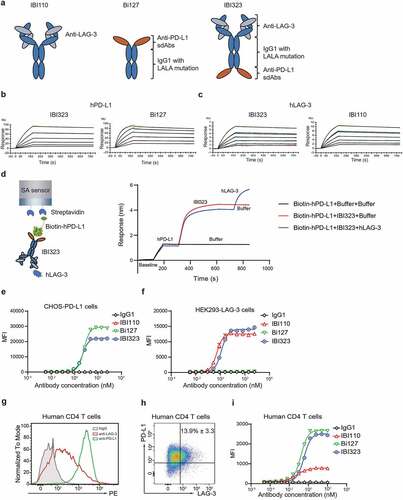
Table 1. Affinity of IBI323, IBI110 and Bi127 to human PD-L1 and LAG-3 measured by SPR
Figure 2. IBI323 activates T cells by blocking the interaction of PD-L1/PD-1 and LAG-3/MHC-II. (a) IBI323 completely blocks the interaction of PD-1 with PD-L1 expressed on CHO-S cells. Cell-based blocking assay was conducted for IBI323, Bi127, and IgG using PD-L1-expressing cell line and PD-1-Fc protein. After incubation and washing, PD-1-Fc was detected by anti-human Fc-PE secondary antibody. (b) IBI323 completely blocks the interaction of CD80 with PD-L1 expressed on CHO-S cells. (c) IBI323 completely blocks the interaction of LAG-3 with MHC-II expressing CHO-S cells. (d) IBI323 blocks PD-1/PD-L1 interaction and promotes T-cell activation in a PD-L1 blockade reporter assay. Jurkat T cells engineered to express human PD-1 with a luciferase reporter driven by an NFAT response element were co-cultured with CHO-K1 cells expressing PD-L1 and an artificial T cell receptor activator. Serially diluted IBI323, IBI110, Bi-127, or IgG1 control was added and luminescent signal was measured after 6 h. (e) IBI323 blocks LAG-3/MHC-II interaction and promotes T-cell activation in a blockade reporter assay. Jurkat T cells engineered to express LAG-3 with a luciferase reporter driven by an NFAT response element were co-cultured with APCs expressing MHC-II in the presence of HA-peptide. Serially diluted IBI323, IBI110, Bi-127, or IgG1 was added and luminescent signal was measured after 6 h. All the data are representative of at least three independent experiments
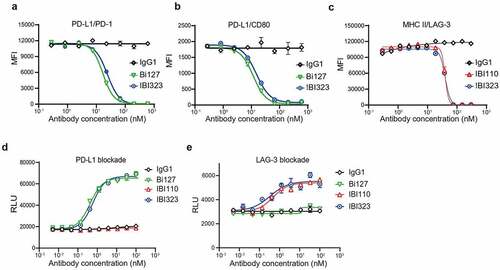
Figure 3. IBI323 generates clustering of cells and enhances primary T-cell activation. (a) IBI323, IBI110, Bi127, or IgG was added to a 1:1 mix of Violet-labeled PD-L1-expressing CHO-S cells and Far Red-labeled LAG-3-expressing 293-F cells. The formation of cell complexes was determined by flow cytometry. Mean percentages of double positive events are shown for each treatment group. Data are representative of three independent experiments. (b) Representative flow plot showing the percentage of double positive cell complexes. (c) Dual blockade of PD-L1/LAG-3 pathway with IBI323 enhances CD4 + T-cell function in an allogeneic MLR. IFN-γ and IL-2 secretion in culture was measured after co-culturing CD4 + T cells with monocyte-derived DCs for 3 days with IBI323, IBI110, Bi127, or IgG1. Data are derived from human PBMC from 2 healthy donors
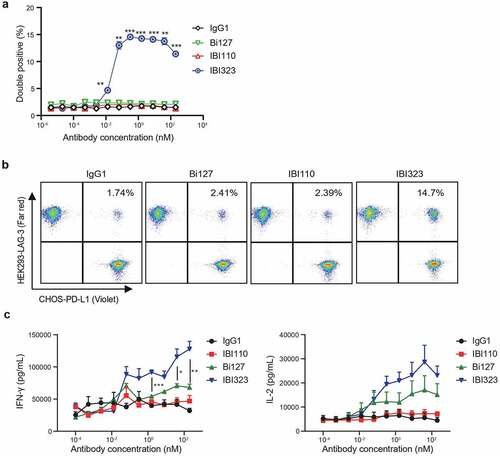
Figure 4. Better tumor control of IBI323 treatment in vivo. Tumor growth (a) and survival (b) of MC38 tumor-bearing mice treated with equalmolar amounts of IBI110 (5.0 mg/kg), Bi-127 (2.6 mg/kg), IBI323 (5.8 mg/kg), IgG (5.0 mg/kg) and a relatively lower dose of IBI323 (1 mg/kg). MC38 cells overexpressing human PD-L1 were subcutaneously injected into human PD-L1/LAG-3 knock-in C57BL/6 mice. Mice were treated with indicated antibodies on days 6, 10, 14, 17, and 21 post implantation. (c) Individual tumor volumes in each treatment group on day 21, the last time point when all animals in the study were alive (n ≥ 5 mice/group). (d, e) Tumor growth and body weight change of NOG mice bearing A375 human melanoma tumor which were treated with IgG (11.6 mg/kg) and IBI323 (3.5 mg/kg or 11.6 mg/kg) (n ≥ 5 mice/group). (f) Tumor growth of NOG mice bearing established A375 human melanoma tumor which were treated with IgG, IBI110 + Bi-127 and IBI323 (11.6 mg/kg or 23.2 mg/kg) (n ≥ 5 mice/group). Statistical analysis was done using two-way ANOVA for growth curves, log-rank test for survival curves, and t test for tumor weights
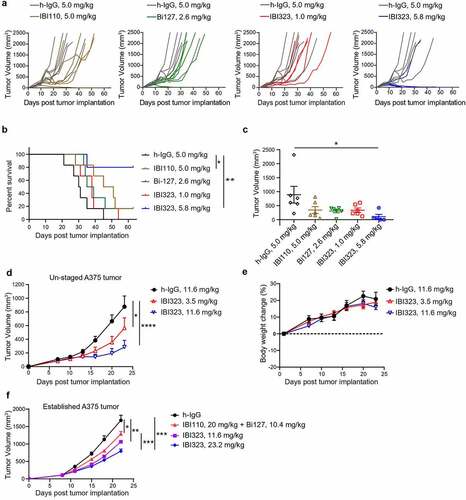
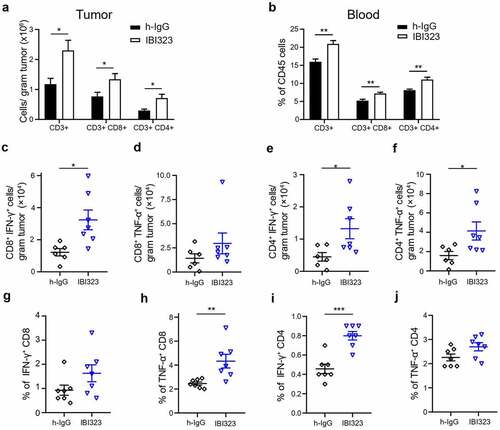
Figure 5. IBI323 treatment dramatically enhances tumor-specific CD8 + T cell response in human PD-L1/LAG-3 knock-in mice bearing human PD-L1-expressing MC38 tumors. Mice were injected with IgG and IBI323 at day 7, 10 and 14 post tumor cell implantation. At day 15, tumor and blood were collected and analyzed by flow cytometry for the absolute counts of the indicated cell subsets in tumor (a) and the proportions of indicated cell subsets within CD45+ cells in blood (b). (c–f) Absolute number of IFN-γ- and TNF-α- expressing T cells from the tumor were quantified, following ex vivo stimulation by MC38 cells. (g–j) Percentage of T cells expressing IFN-γ and TNF-α following ex vivo stimulation by MC38 cells in blood