Figures & data
Figure 1. Tumoral-secreted IL-1α promotes tumor growth in murine HCC models.
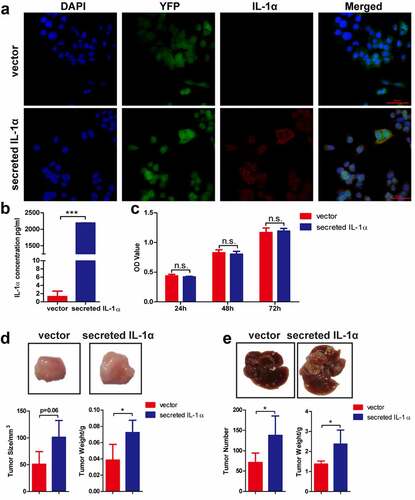
Figure 2. Tumoral-secreted IL-1α inhibits T and NK cell activation in vivo.
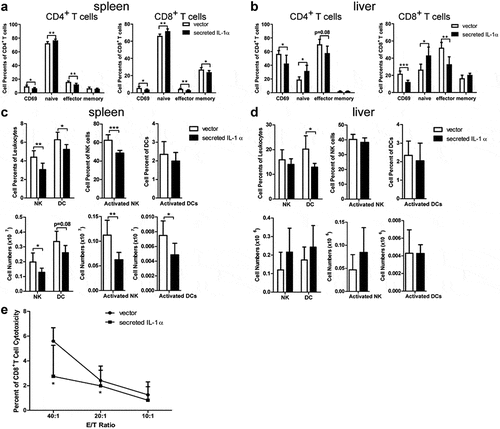
Figure 3. Tumoral-secreted IL-1α increases MDSCs in vivo.
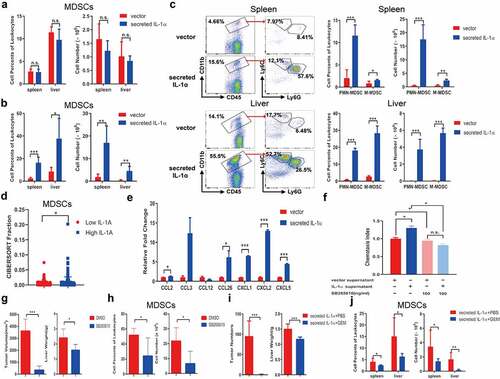
Figure 4. Systemic administration of recombinant IL-1α inhibits tumor development.
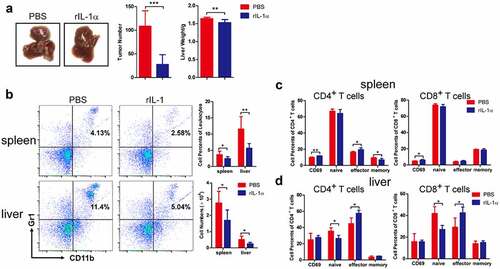
Figure 5. Secreted IL-1α promotes T cell activation in vitro.
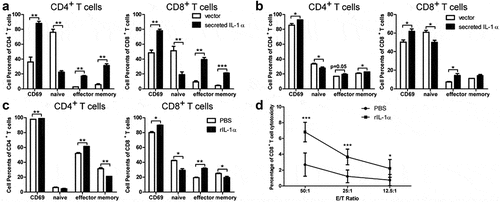
Figure 6. Calpain 1 is essential for tumoral-secreted IL-1α-mediated HCC progression.
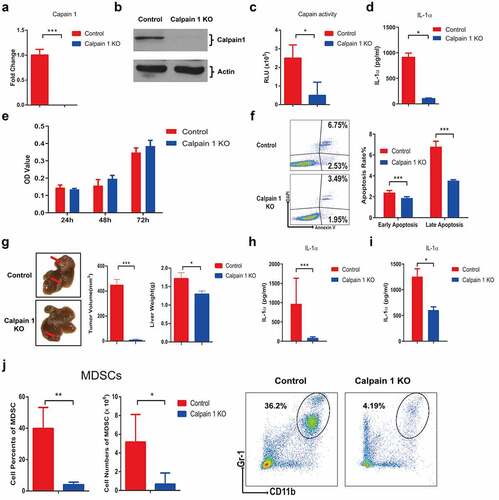
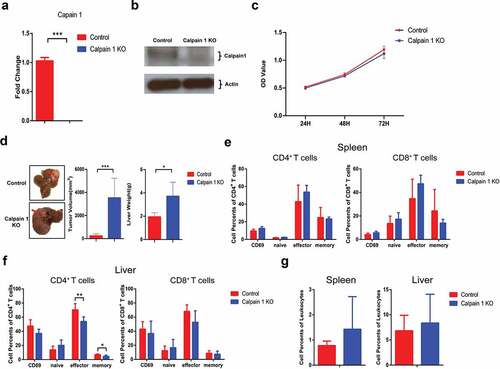
Figure 7. Calpain 1 KO promoted HCC development in parental hepa1-6 cells.