Figures & data
Figure 1. Schematic of HSN and PVQ neurons in sdn-1 and trp mutants. In wild type worms, the HSN neurons migrate from the posterior to mid-body during embryogenesis. Axon extension occurs during larval development where the HSN neurons extend axons ventrally and around vulva before entering the ventral nerve cord. A hypodermal ridge separates HSNL and HSNR neurons. In sdn-1 (zh20) mutants, the HSN cell bodies exhibit defective migration and their axons are misguided. However, abolishing trp-1 and trp-2 in sdn-1 mutant animals partially restores HSN development to wild type. Similarly, the PVQ neurons extend anteriorly directed axons from the tail. The axons are separated by the hypodermal ridge and extend to the nerve ring with minimal crossover events. PVQ axons are misguided in sdn-1 mutant animals where they exhibit frequent crossovers to the contralateral side of the ventral nerve cord. These defects could be ameliorated by removal of trp-1 and trp-2.
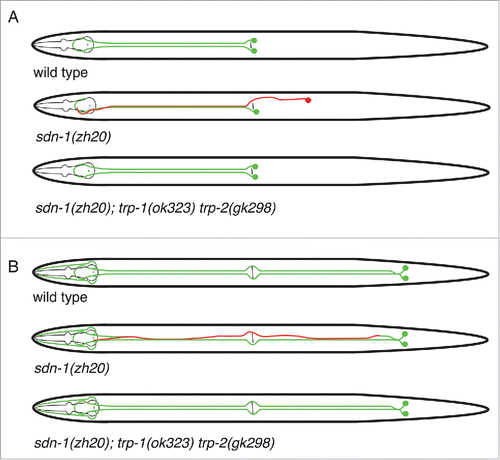
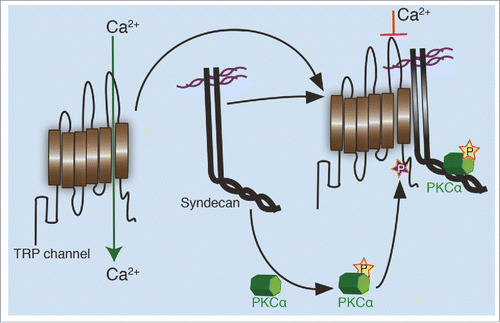
Figure 2. Regulation of TRP channels by syndecans. In an unphosphorylated state, TRP channels allow calcium transport through the plasma membrane. When aligned in close proximity with a syndecan, possibly through complex formation between the syndecan and TRP channel, an active PKCα created during the interaction, phosphorylates the channel on a conserved serine residue. This phosphorylation closes the channel formed between 5th and 6th transmembrane domain, limiting the cellular calcium influx.