Figures & data
Figure 1. CHN-1 defines insulin signaling and longevity through DAF-2 ubiquitylation. (A) In the absence of stress, (1) CHN-1 binds to and monoubiquitylates the DAF-2/insulin receptor in collaboration with an E2 enzyme (e.g. LET-70),Citation24 which triggers (2)-(3) endocytic-lysosomal degradation of the insulin receptor.Citation6 Reduced insulin signaling supports nuclear localization of the transcription factor DAF-16 and expression of pro-longevity genes. (B) Stress- or aging-related decline in proteostasis cause increased level of misfolded proteins, which redirects CHN-1 activity toward chaperone-assisted ubiquitylation. The stabilization of membrane-bound DAF-2 triggers insulin signaling, which limits nuclear translocation of DAF-16 and consequently shortens lifespan.
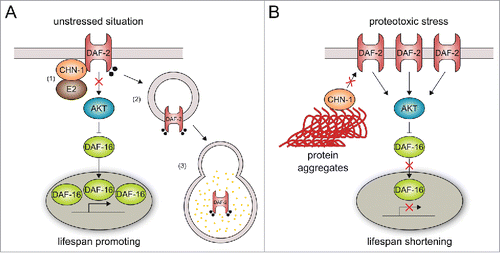
Figure 2. Proteostasis collapse redirects substrate specificities of the proteolytic network. Stress and aging induce a rearrangement of protein quality control networks, including molecular chaperones and protein degradation pathways, to maintain proteostasis. Among other quality control factors, this process recruits the E3 ubiquitin ligase CHN-1 and co-working chaperone Hsp90 to protein aggregates for chaperone-assisted ubiquitylation of misfolded proteins. Consequently, the activity of natural CHN-1 substrates remains uncontrolled, which provokes pathologies and further accelerates aging.