Figures & data
Table 1. Demographic data for the ME/CFS patients at initiation of treatment.
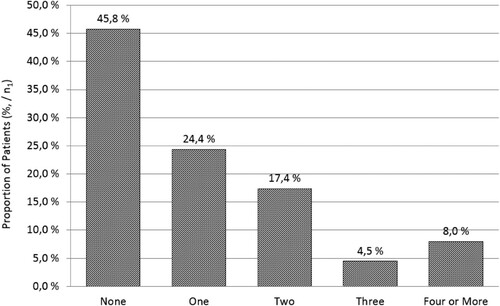
Table 2. Most common treatment responses to LDN therapy in ME/CFS (N = 218).
Table 3. Prevalence of adverse symptoms during the initial phase of LDN treatment (N = 201).
Table 4. Adverse symptoms that occurred rarely (2% or less) during the initiation of LDN (N = 201). These symptoms are common in untreated ME/CFS population.
Table 5. Adverse symptoms that were reported only once (0.5%/N = 201). Connection with LDN therapy unsure.
Table 6. Nausea was the most common reason for discontinuing LDN. Altogether, 15 patients (7.5%) discontinued LDN due to adverse symptoms (N = 201).
Figure 1. The follow-up periods of individual patients who responded to LDN and continued with the treatment. The average follow-up period was 1.7 years (0.1–6.8 years). 91 patients (63%) were followed up for more than one year.
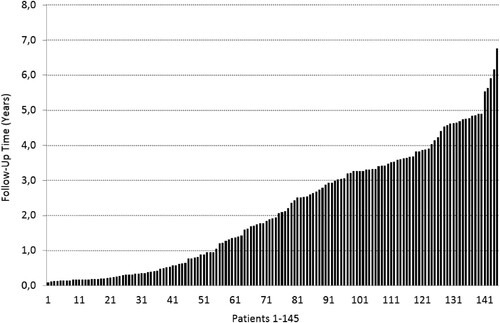
Figure 2. LDN alleviated the symptoms of ME/CFS in a minimum of 73.9% of the patients. Despite symptom alleviation, 1.8% discontinued with LDN because of adverse symptoms. 18.3% were non-responders. In a subset of them (5.5% of the entire cohort) the discontinuation was caused by adverse symptoms (). 7.8% of patients were lost to follow-up. These patients were included in the non-responder group.
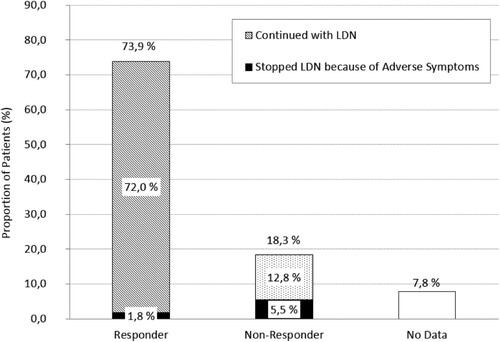
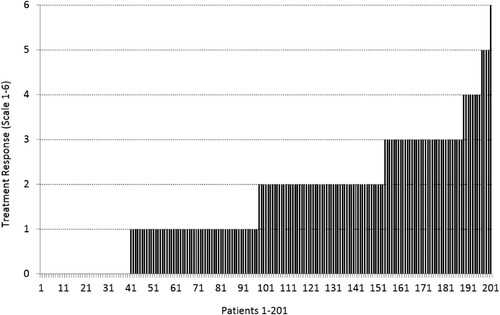
Figure 3. The quantified treatment response in 201 patients on a scale of 0–6. Points (0–5) were scored for improved vigilance/alertness, improved physical performance, improved cognition, pain reduction, less fever. One additional point was scored if the patient experienced improvement in some (one or more) other ME/CFS symptoms of ME/CFS. Patients 1–40 (18.3%) were non-responders.