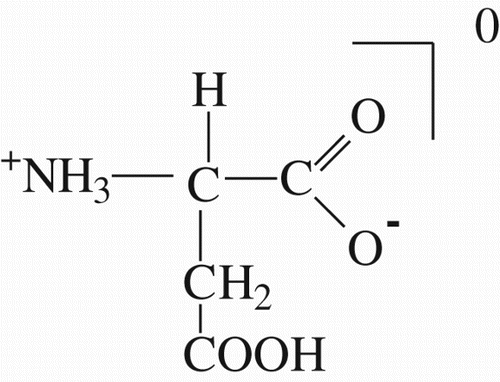
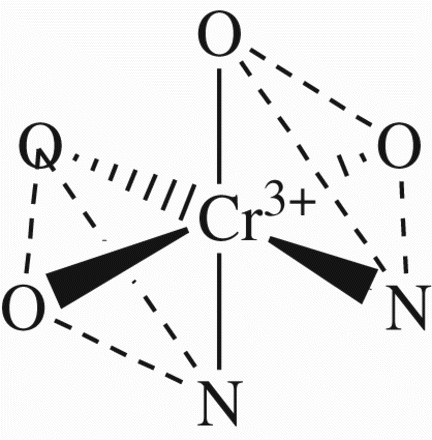
Figures & data
Figure 1. (a) Four potentiometric titrations of free Asp in 0.1 M NaNO3, 25°C. Sharp inflections separate the ammonium proton from the β-carboxylic acid proton. The insets are (b) correlation of the volume of added titrant in mL vs. potential in mV, and (c) is the correlation of measured pH values (pHobs) vs. potential in mV.
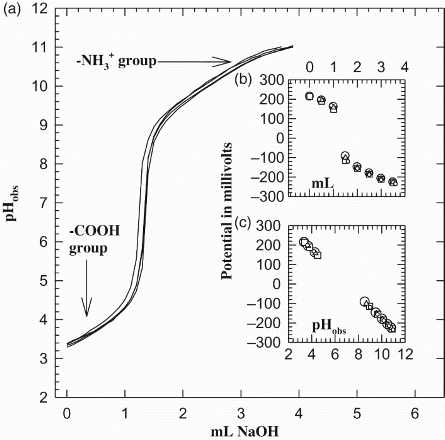
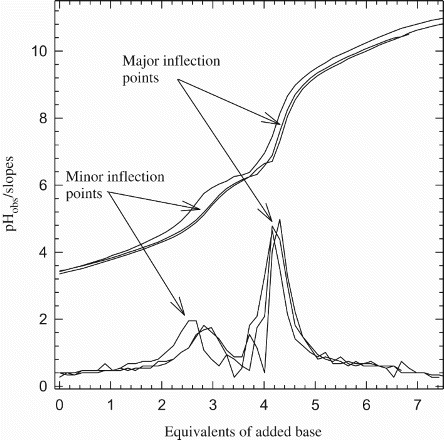
Figure 2. Representative graph of potentiometric titrations of Cr3+: Asp in 1:2 ratio in 0.1 M NaNO3, 25°C. The 1:1 and the 1:3 ratios show similar titration patterns.

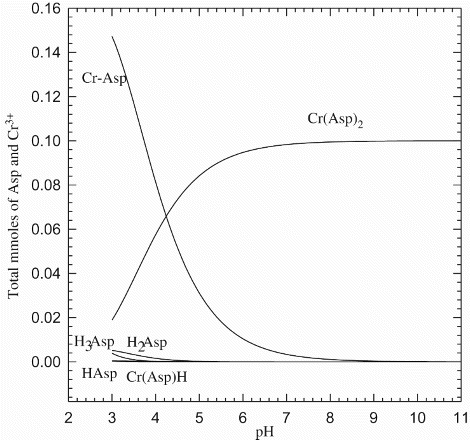
![Figure 4. UV–Vis absorption spectra of Cr3+: Asp in 1:2 ratio. [Cr3+]=1.9×10−4 M. Equilibrium time was set to 50 days.](/cms/asset/4f3c710a-6909-4310-84f1-17bc31eb6797/tcme_a_883291_f0004_b.gif)