Figures & data
Figure 1. Subject distribution between the adult-adolescent substudy and the lot consistency substudy. The V503-002 study (NCT #00943722) was designed to enroll 1800 girls 9 to 15 years of age, 600 boys 9 to 15 years of age, and 400 young women 16 to 26 years of age. The study was comprised of two immunogenicity substudies: 1. An adult-adolescent immunobridging substudy to compare 9vHPV vaccine immunogenicity at Month 7 in girls versus young women, and boys versus young women; 2. A lot consistency substudy to demonstrate consistent immunogenicity at Month 7 in subjects randomized to three different vaccine lots of the final manufacturing process.
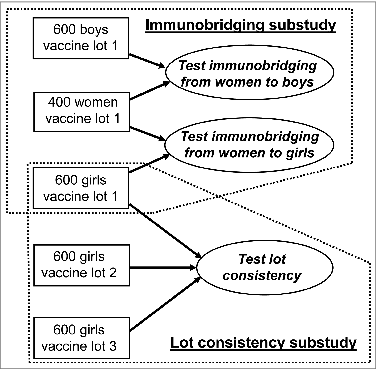
Table 1. Subject characteristics
Figure 2. Accounting of subjects in the lot consistency substudy. A total of 1935 subjects were randomized from 65 sites in Africa (South Africa), Asia (India, Korea, Taiwan, Thailand), Europe (Austria, Belgium, Finland, Poland, Sweden, Spain), Latin America (Brazil, Chile, Colombia, Costa Rica, Peru) and North America (United States). Withdrawal = patient withdrew consent.
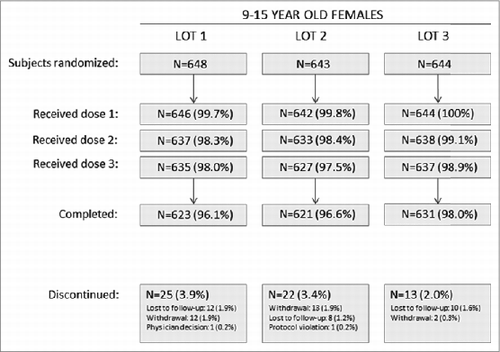
Table 2. Statistical analysis of equivalence of geometric mean titers at month 7
Table 3. Statistical analysis of equivalence of proportions of subjects who seroconverted by month 7
