Figures & data
Figure 1. Participant flow diagram. Footnote to figure: 5/10 = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μgAT; 10 μg ClfA 5/10AS = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μg AT; 10 μg ClfA adjuvanted with AS03B 10/30 = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA 10/30AS = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA adjuvanted with AS03B TVC, total vaccinated cohort; ATP, according-to-protocol; N, number of subjects in a category. *Contraindications to subsequent vaccination: clinically relevant abnormal laboratory value (26), interval between dose 3 visit and visit before out of delay (>15 days) (2) and alcoholism (1). Laboratory values included: hematology (red blood cell count, white blood cell count, eosinophils count, neutrophils count, lymphocytes count, platelets count, reticulocytes index, hemoglobin), biochemistry (ALT, AST, creatinine, LDH, CPK, total bilirubin) and urinalysis (protein, glucose and blood).
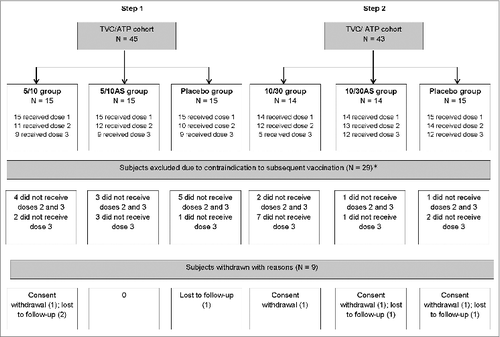
Figure 2. The incidence of solicited local (A) and general (B and C) adverse events reported during the 7-day post-vaccination period (total vaccinated cohort) Footnote to figure: 5/10 = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μgAT; 10 μg ClfA 5/10AS = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μg AT; 10 μg ClfA adjuvanted with AS03B 10/30 = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μgAT, 30 μg ClfA 10/30AS = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA adjuvanted with AS03B.
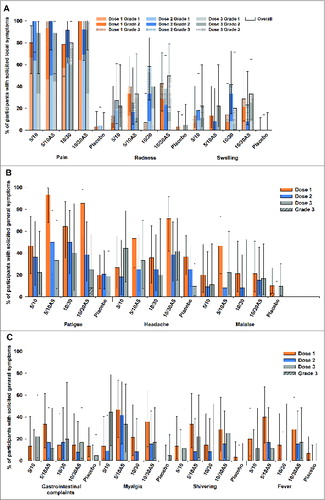
Figure 3. Geometric mean concentrations of anti-capsular polysaccharide types 5 (panel A) and 8 (panel B), anti-AT (panel C) and anti-ClfA antibodies (panel D) (according-to-protocol cohort for immunogenicity) Footnote to figure: 5/10 = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μg AT; 10 μg ClfA 5/10AS = 5 μg CPS5-TT; 5 μg CPS8-TT; 10 μg AT; 10 μg ClfA adjuvanted with AS03B 10/30 = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA 10/30AS = 10 μg CPS5-TT; 10 μg CPS8-TT; 30 μg AT; 30 μg ClfA adjuvanted with AS03B 95% CI; 95% confidence interval; GMC, geometric mean concentration; AT; α-toxin; CPS5 and 8, capsular polysaccharides types 5 and 8; TT, tetanus toxoid; ClfA, clumping factor A; L.U, Luminex units; EL.U, ELISA units. PRE, pre-dose 1; PI(D7), 7 d post-dose 1; PtdIns(D14), 14 d post-dose 1; PI(D30), 30 d post-dose 1; PII(D37), 7 d post-dose 2; PII(D44), 14 d post-dose 2; PII(D60), 30 d post-dose 2; PII(D179), pre-dose 3; PIII(D187), 7 d post-dose 3; PIII(D194), 14 d post-dose 3; PIII(D210), 30 d post-dose 3; PIII(D360), 1 y post-dose 1 or 6 months post-dose 3; PIII(D540), 1.5 y post-dose 1 or 1 y post-dose 3 The cut-off values for these assays (dashed line) were 23.6 L.U/mL for CPS5, 26.5 L.U/mL for CPS8, 22.5 L.U/mL for AT and 6 EL.U/mL for ClfA. Blue arrows indicate days of vaccine or placebo administration. *Only 4 subjects were included in the analysis of ClfA antibody levels in the 10/30 group from PIII(D187) onwards. This explains the large 95% CI for these time points.
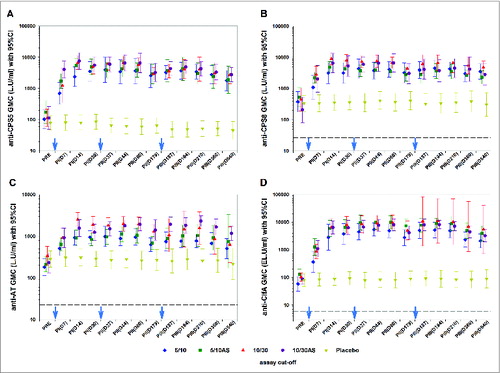
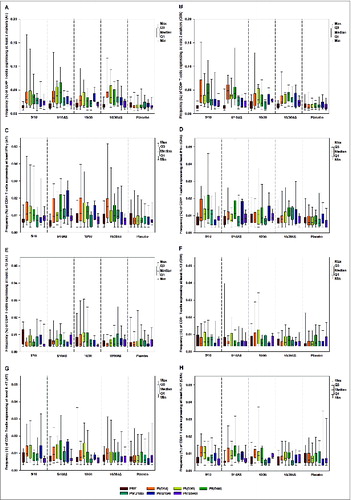
Figure 4. Frequency (%) of S. aureus AT-specific (panels A, C, E and G) and ClfA-specific (panels B, D, F and H) CD4+ T-cells expressing at least 2 markers among IL-2, IFN-γ, IL-13, IL-17, TNF-α and CD40L (panels A and B), at least IFN-γ (CD4-Th1 profile) (panels C and D), IL-13 (panels E and F) and IL-17 (panels G and H) prior and after each vaccination (according-to-protocol cohort for immunogenicity) Footnote to figure: 5/10 = 5 μg CPS5-TT, 5 μg CPS8-TT, 10 μg AT, 10 μg ClfA 5/10AS = 5 μg CPS5-TT, 5 μg CPS8-TT, 10 μg AT, 10 μg ClfA adjuvanted with AS03B 10/30 = 10 μg CPS5-TT, 10 μg CPS8-TT, 30 μg AT, 30 μg ClfA 10/30AS = 10 μg CPS5-TT, 10 μg CPS8-TT, 30 μg AT, 30 μg ClfA adjuvanted with AS03B AT, α-toxin; ClfA, clumping factor A; PRE, pre-dose 1; PtdIns(D14), 14 d post-dose 1; PI(D30), 30 d post-dose 1; PII(D44), 14 d post-dose 2; PII(D179), pre-dose 3; PIII(D194), 14 d post-dose 3; PIII(D540), 1.5 y post-dose 1 or 1 y post-dose 3; Min/Max, Minimum/Maximum; Q1,Q3, First and third quartile.