Figures & data
Table 1. Overview of phase IIB and III trials of prophylactic HIV vaccine strategies
Figure 1. Schematic illustration of different trial designs. (A) Multi-arm design, Example of a design, comparing 3 different vaccine strategies to a common placebo arm. (B) Two-by-2 factorial 4-arm design. Example of a design evaluating a vaccine strategy, another intervention and the combination of both. The other intervention could for instance be pre-exposure prophylaxis (PrEP) in case of a HIV prevention trial or a drug to mobilize the viral reservoir in case of a therapeutic trial. (C) Two-arm group-sequential design Example of a design with one interim analysis and stopping rule (D) Seamless adaptive design. Example of an adaptive design with a seamless progression between phase II and phase III, integrating a selection of trial arms during phase II and additional design adaptations at the beginning of phase III.
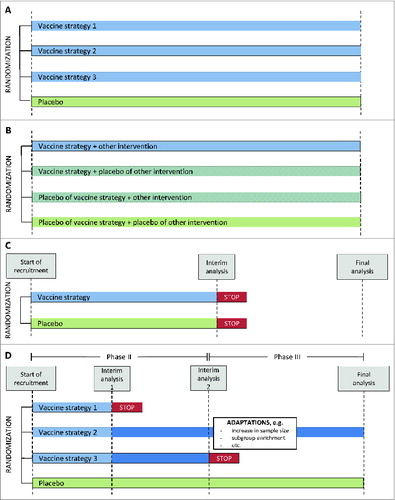
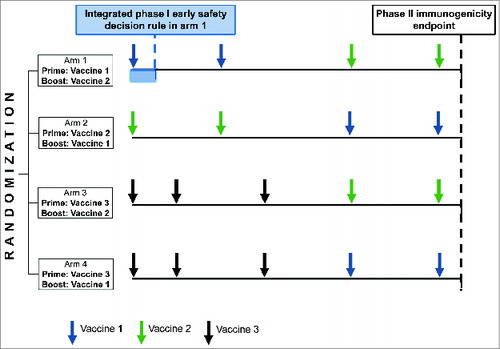
Figure 2. Illustration of a multi-arm phase I-II design evaluating 4 vaccine strategies in parallel and integrating an early safety decision rule for one of the vaccines (vaccine 1).