Figures & data
Table 1. Clinical trials for 3 human Enterovirus 71 (EV71) vaccines
Table 2. The change of EV71 and CA16 NTAbs in infants and children in clinical trial 1, 2 and 3
Table 3. The change of EV71 and Poliovirus NTAbs in infants and children from clinical trial 4
Table 4. The seropositive ratios of NTAb for inactivated-EV71 vaccination after CA16 or Poliovirus vaccination
Figure 1. The NTAb GMTs for inactivated-EV71 vaccination after CA16 or Poliovirus vaccination. BALB/c mice (n = 10 per group) were subcutaneously injected with CA16 virus (VR18, genebank no:JX481738, 4.8 × 105 PFU/mouse), inactivated-Poliovirus vaccine (Sanofi Pasteur, lot:G0510-1, Type I 20 DU/mouse, Type II 4 DU/mouse, Type III 16 DU/mouse), or aluminum adjuvant (Adjuvant). All animals were boosted in week 3 after priming. One week after the boost, all mice took the first inactivated-EV71 vaccination (SINOVAC BIOTECH CO.,LTD., 200 U/mouse), followed by the second inactivated-EV71 vaccination 3 weeks later. Negative control group was just inoculated with saline. Sera were collected on day 28 (7 days after the 2nd boost) and day 56. All the sera were stored at −20°C. Neutralization titers (NTs) of the sera for EV71 NTAb, CA16 NTAb and Polio I, II, III NTAb were determined. Data were expressed as means ± SEM. “Pre” and “Post” are equal to before (on 28 day) and after (on 56 day) EV71 vaccination. For analysis of GMTs, the data were transformed using the log 2 of the original values. Panels a-e separately show CA16, Polio I-III and EV71 neutralization titers for each group before and after EV71 vaccination and panel f shows immunization design for this experiment. Note: * means the GMTs were significantly different after vaccination (P < 0.05).*** means the GMTs were very significantly different after vaccination (P < 0.0001).
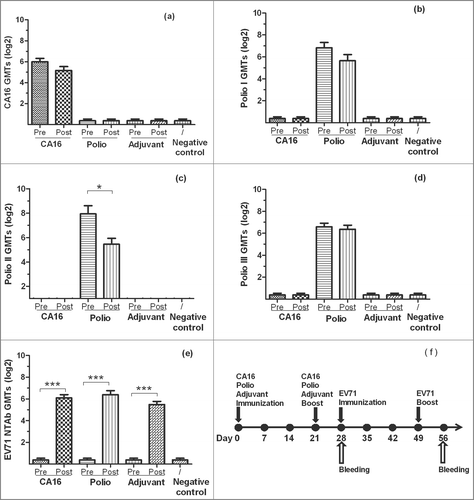
Table 5. The Seropositive ratio of NTAb for inactivated-EV71 vaccine co-immunized with CA16 or Poliovirus
Figure 2. The NTAb GMTs for inactivated-EV71 vaccine co-immunized with CA16 or Poliovirus vaccine. 60 pathogen-free BALB/c mice (6–8 weeks,female,purchased from Vital River Lab Animal Technology Co., Ltd, Beijing, China) were used. BALB/c mice (n = 10 per group) were subcutaneously injected with inactivated-EV71 vaccine (SINOVAC BIOTECH CO.,LTD., 200 U/mouse), CA16 virus (VR18, genebank accession no:JX481738, 4.8 × 105 PFU/mouse), inactivated-Poliovirus vaccine (Sanofi Pasteur, lot:G0510-1, Type I 20 DU/mouse, Type II 4 DU/mouse, Type III 16 DU/mouse), or co-immunized with inactivated-EV71 vaccine (EV71 & CA16 group and EV71 and polio group). The control group was just inoculated with aluminum adjuvant (Adjuvant). The animals were boosted in week 3 after priming. All the sera were collected one week after the boost and stored at −20°C. Neutralization titers (NTs) of the sera were determined for EV71 NTAb, CA16 NTAb and Polio I, II, III NTAb. Data were expressed as means ± SEM. For analysis of GMTs, the data were transformed using the log 2 of the original values. Panels a-e separately show EV71, CA16 and Polio I-III neutralization titers for each group, and panel f shows immunization design for this experiment. Note: *** means this group were significantly different when compared with other groups without *** label (P < 0.0001).
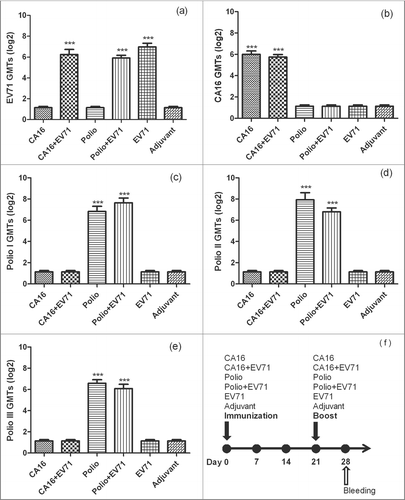
Table 6. Homology of 2 EV71 neutralizing epitopes with CA16 and poliovirus
