Figures & data
Table 1. Characteristics of randomized study participants
Table 2. Antigen and adjuvant composition of vaccines administered
Table 3. Immunogenicity analyses: HPV 6, 11, 16, and 18
Figure 2. Anti-HPV31, 45, 52, and 58 GMTs in Study 1 at one month post-dose 3; B) Interim analysis of anti-HPV31, 33, 45, 52, and 58 GMTs in Study 2 at one month post-dose 2.
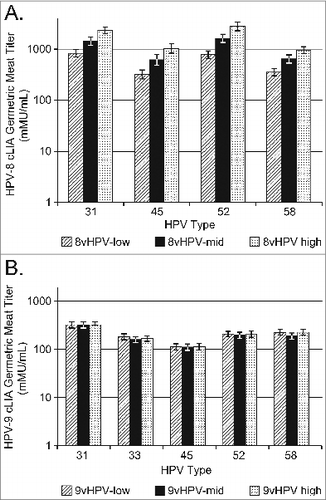
Table 4. Adverse event (AE) summary