Figures & data
Table 1. Demographic characteristics (ATP cohorts for immunogenicity)
Figure 1. Trial profile. Withdrawals from the study: Primary phase, PHiD-CV group; allergic reaction to the study vaccines of grade 1 intensity (one child), SAE (Kawasaki's disease, one child), simultaneous participation in another clinical trial (one child), sudden infant death syndrome (one child). Primary phase, control group: move from the study area (one child). Booster phase, PHiD-CV group: consent withdrawal not due to an AE (one child), move from the study area (one child).
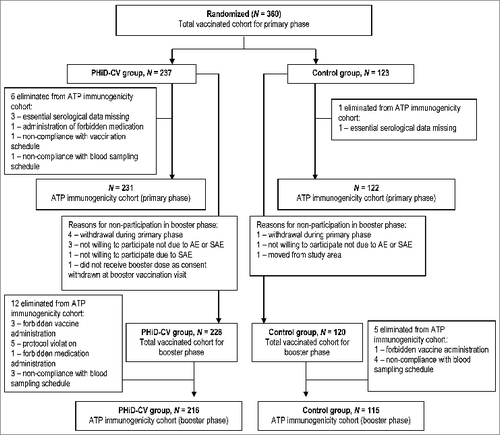
Table 2. 22F-ELISA antibody geometric mean concentration (GMC) ratios between pivotal immunologic non-inferiority PHiD-CV study in Europe and PHiD-CV study in Japan one month after the third vaccine dose (ATP cohort for immunogenicity)
Table 3. Opsonophagocytic activity (OPA) geometric mean titer (GMT) ratios between 11Pn-PD/acute otitis media efficacy study in Europe (POET) or PHiD-CV/pneumococcal diseases efficacy study in Latin America (COMPAS) and PHiD-CV study in Japan one month after the third vaccine dose (ATP cohort for immunogenicity)
Table 4. 22F-ELISA antibody and opsonophagocytic activity (OPA) seropositivity rates for individual pneumococcal serotypes following vaccination with PHiD-CV co-administered with DTPa. Pre-vaccination and post-priming data are for ATP immunogenicity cohort for primary vaccination. Pre-booster and post-booster data are for ATP immunogenicity cohort for booster vaccination
Figure 2. 22F-ELISA antibody geometric mean concentrations (GMCs) or opsonophagocytic activity (OPA) geometric mean titers (GMTs), with 95% confidence intervals, against individual pneumococcal serotypes before and one month after vaccination with PHiD-CV co-administered with DTPa (logarithmic scale, ATP cohorts for immunogenicity). Pre-vacc, before the first dose (at approximately 3 months of age); Post-priming, one month after 3-dose priming (at approximately 6 months of age); Pre-booster, before booster dose (17 to 19 months of age); Post-booster, one month after booster dose (18 to 20 months of age).
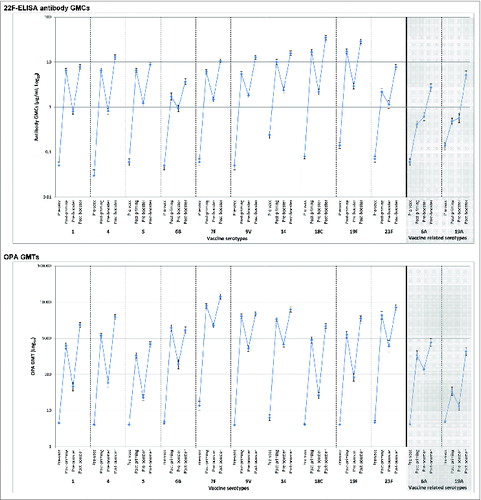
Figure 3. Study design. *Children in the control group were allowed catch-up vaccination with 7vCRM (2 doses administered between the second blood sampling time point and 7 d before the DTPa booster dose). Children in both groups were allowed to receive Haemophilus influenzae type b (Hib) and hepatitis b virus (HBV) vaccines concomitantly with the study vaccines. Administration of Bacille Calmette-Guérin, oral polio, measles-rubella, varicella and mumps vaccines was allowed, according to local recommendations, up to 28 d before or at least 7 d after DTPa or PHiD-CV administration.
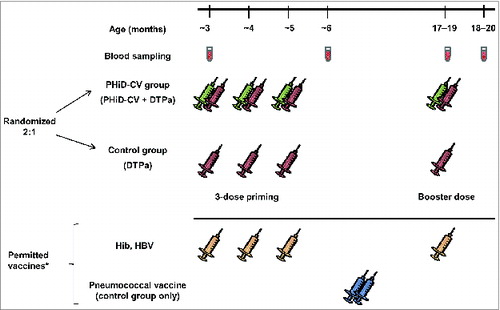
Table 5. Incidence of solicited local symptoms at each injection site and solicited general symptoms within 8 d (days 0–7) after each vaccine dose (total vaccinated cohorts)
