Figures & data
Figure 1. Mouse study reveals that excess IC31 is necessary for modulation of the H4 response. H4-specific IFNγ response was measured in Day 36 splenocytes after 2 intramuscular (IM) immunizations in right and left quadriceps (Day 0 “priming” and Day 21 “boost”) with H4-IC31 formulations. Formulations were prepared prior to each injection by mixing 2 concentrations of H4 (50 µg/mL or 1µg/mL) with serial dilutions of IC31 in a 1:4 volumetric ratio on a rotator. The total volume of formulations used for each injection was 50 µL. Statistical analysis was performed with the SAS® Software version 9.2 at an α risk of 5% for the main effects and 10% for the interaction effects. Comparison of H4-IC31 formulations with the minimal formulation was performed based on an ANOVA model. A mixed model with IC31 doses (log10) as fixed factors was used with Dunnett adjustment. Bars represent the geometric mean titers of IFNg responses in each group. White circle symbols represent individual IFNg responses in each group.
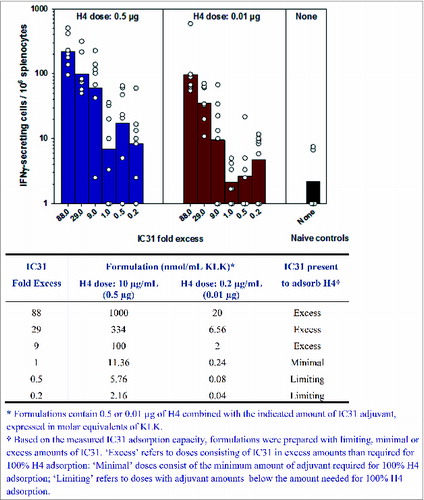
Figure 2. Modulation of H4 response by IC31 in hWB over an extended incubation period. Fresh blood was collected into heparinized 10 mL tubes from selected healthy BCG-vaccinated or Tuberculosis Skin Test positive volunteers. H4-IC31 formulations were prepared by mixing H4 (diluted in serum-free medium to 1 µg/mL, 0.1 µg/mL, or 0.01 µg/mL) with IC31 (diluted in serum-free medium to 10 nmol/mL) in a 1:4 volumetric ratio on a rotator. The mixed product was shown to remain stable for up to 24 hours at room temperature. Upon preparation, formulations were immediately added in 6 replicates on 96-well, U-bottom plates (100 µL/well). hWB (10-fold diluted) was mixed freshly with the formulations on the plates (within 24 hours of plating the formulations), resulting in an additional 2-fold dilution of the blood and formulations. IFNγ responses were measured at time points of Day 6 (D6) or Day 12 (D12) in supernatants of treated hWB cultures. For each formulation containing IC31, 2 observations were made per subject as 2 IC31 lots were tested per formulation. Statistical regressions were performed with JMP Statistical Discovery Software version 7.0.1 using ordinary least squares with a Restricted Maximum Likelihood (REML) mixed model. Differences in immune responses were considered to be significant when p values of less than 0.05 were obtained: (A) D6 and D12 responses to H4-IC31 formulations containing a constant concentration of IC31 and 3 titrations of H4. H4-TLR4-Adjuvant was used as an internal positive control. The cultures were harvested and replenished on D6 with serum-free medium (n = 4); (B) D12 responses to H4 antigen at 3 dosages formulated with or without IC31 adjuvant. The cultures were not harvested or replenished on D6 for the last 3 subjects (n = 7).
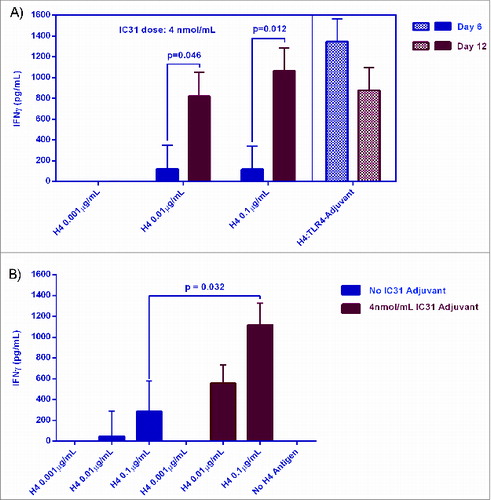
Figure 3. hWBA confirms the importance of excess IC31 for the optimal modulation of H4 response. ELISA analysis of IFNγ responses measured in supernatants of hWB cultures 10 d post-treatment with H4-IC31 formulations containing a constant dose of H4 and various doses of IC31 (n = 5). Formulations were prepared by mixing H4 (5 µg/mL) with serial dilutions of IC31 in a 1:4 volumetric ratio on a rotator. This was followed by a 10-fold dilution of formulations in serum-free medium. Upon preparation, formulations were immediately added in 6 replicates on 96-well, U-bottom plates (100 µL/well). hWB (10-fold diluted) was mixed freshly with the formulations on the plates (within 24 hours of plating the formulations), resulting in an additional 2-fold dilution of the blood and formulations. The cultures were harvested and replenished on D6 with serum-free medium. 'Limiting dose' where adjuvant amount was 0.5 and 0.2-fold below 100% H4 adsorption; 'minimal dose' consisting of minimum amount of adjuvant required for 100% H4 adsorption; and 'excess dose' consisting of IC31 in 9-, 29-, and 70-fold higher than required for 100% H4 adsorption. Controls: H4 (0.05 µg/mL), IC31 (4 nmol/mL), Phytohemagglutinin (PHA, 5 µg/mL).
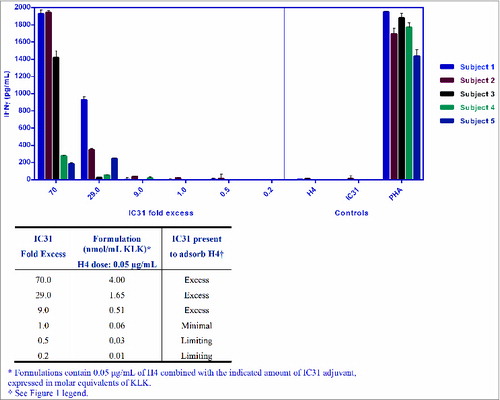
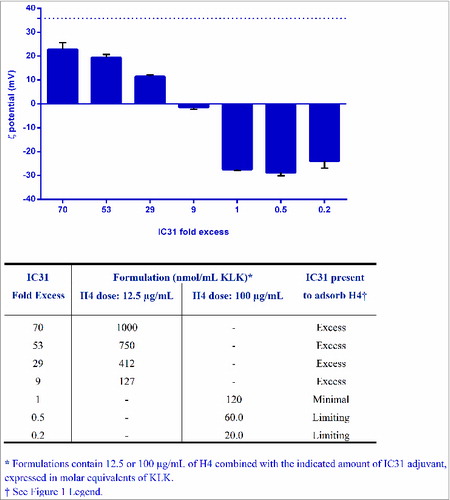
Figure 4. The Surface charge of formulations switches from negative to positive when IC31 is greater than 9 fold in excess. The ζ potential of formulations containing different H4-IC31 ratios expressed as IC31 fold excess was measured by phase analysis light scattering on a ZetaPALS (Brookhaven Instruments, Holtsville, NY). The dotted line represents the ζ potential value of IC31 in the absence of H4.