Figures & data
Figure 1. Levels of anti-Aβ antibodies in sera and brain. (A) Anti-Aβ antibodies in blood samples one week after each vaccination markedly increased and then entered a platform stage after the third vaccination. (B) Anti-Aβ antibodies in brains were higher after the sixth vaccination compared with the third vaccination. The data are presented as the means ± SD (n = 8, *P < 0.05, **P <0.01, *** P < 0.001).
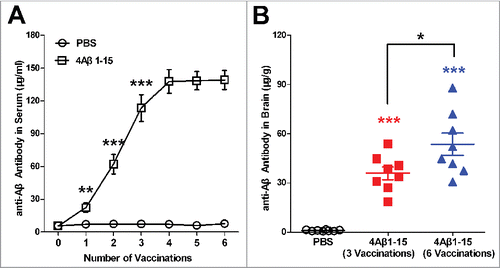
Figure 2. Inflammatory factors in sera and brain. (A) Levels of inflammatory factors in sera present specific fluctuations that first increase and then decrease. (B) Levels of inflammatory factors in the brain present a similar variation trend as in the sera. (C) The standardized ratio of IFN-γ to IL-4 in the brain, representing the balance of Th1/Th2, showed Th1-polarized immune response first and then Th2-polarized immune response compared with the controls. The data are presented as the means ± SD (n = 8, *P < 0.05, **P <0.01, *** P < 0.001).
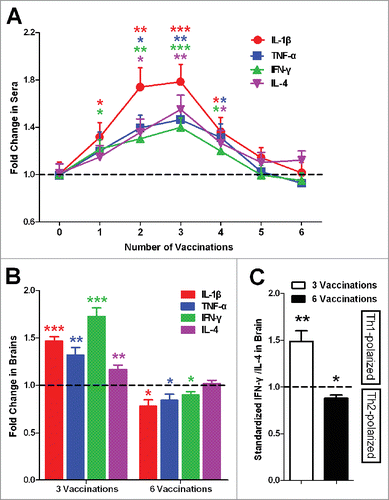
Figure 3. Confocal micrographs of brain sections with immunohistochemical staining represent the cerebral Aβ pathology in APP/PS1 mice after the third injection (A–C) and the sixth injection (D–F). Micrographs of brain sections obtained from mice with the median level of Aβ plaque burden in their respective groups. Scale bars are indicated in the figures. Histograms show the percentages of Aβ burden calculated by quantitative image analysis (n = 8). The reduction percentage relative to the controls is indicated in the figure. More Aβ plaque burdens were cleared after the sixth injection in 4Aβ1-15-treated mice (*P <0.05).
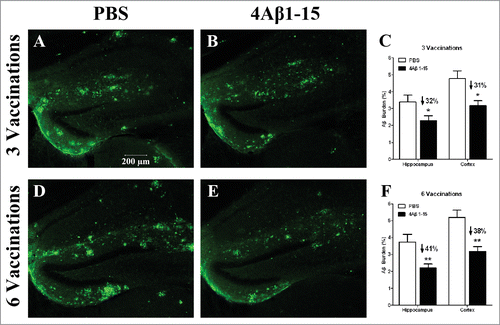
Figure 4. 4Aβ1-15 improves the spatial learning and memory ability in APP/PS1 mice as shown by the Morris water-maze (MWM). After three injections, (A) 4Aβ1-15-treated mice show less escape latency in the mean value, except for the fourth day in the place navigation. Meanwhile, (B) on the 5th day of the spatial probe trial, mice in the 4Aβ1-15 group spent a little larger proportion of time and distance in the target quadrant. Compared with controls, mice injected 6 times present markedly improvement in both place navigation (C) and the spatial probe trial (D) (n = 8, *P < 0.05).
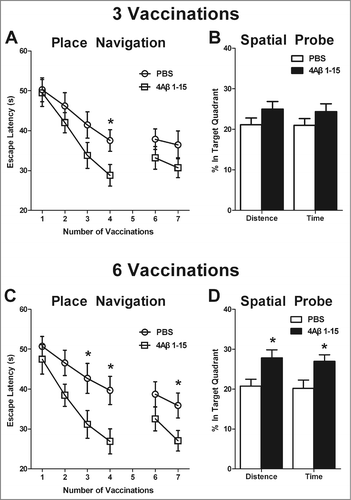
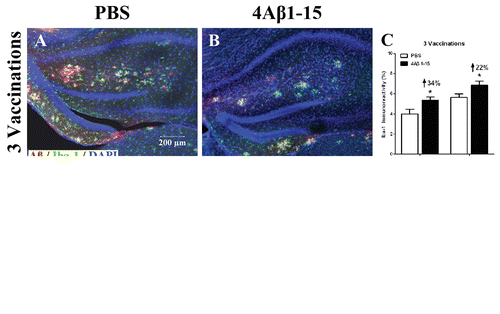
Figure 5. Confocal micrographs show Aβ plaques (red) and activated microglial cells (green) representing the immunoreactivity of Iba-1 positive cells and doubly labeled immunohistochemically. Cell nuclei are labeled with DAPI (blue) (A, B, D, E). Scale bars are indicated in the figures. The microglial are activated into amebocyte morphology and tend to surround Aβ plaques. The histogram shows a larger degree of microglial activation after the third injection (C), whereas it is significantly reduced after (F) compared with the controls. The reduction percentage is indicated in the figure (n = 8, *P < 0.05).