Figures & data
Figure 1. Expression construct for Na-APR-1 (M74). (A) Schematic of the expression construct for Na-APR-1 (M74), and (B) Na-APR-1 (M74) amino acid sequence. The PR-1a signal peptide is shown in bold.
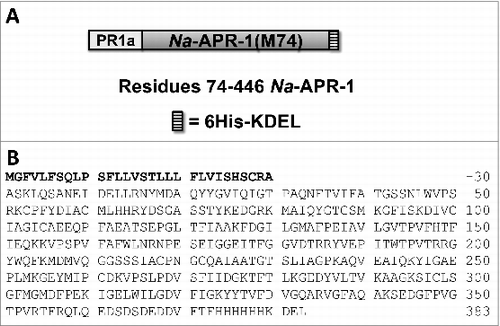
Figure 2. Process Schematic Overview. (A) Schematic of the purification process of Na-APR-1 (M74). (B) Summary of the process steps for the harvest/clarification and for each chromatography step.
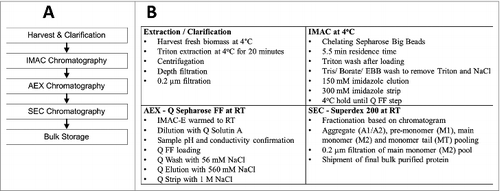
Figure 3. SDS-PAGE analysis of in process samples (A) Coomassie stained SDS-PAGE (10%) of non-reduced purification in process samples; M – molecular weight markers, H – homogenate, I – immobilized metal affinity chromatography (IMAC) eluate, Q – ion exchange chromatography eluent, S – size exclusions chromatography pooled eluent, F – final purified Na-APR-1 (M74). (B) Western blot of non-reduced process samples probed with an anti-APR1 primary antibody.
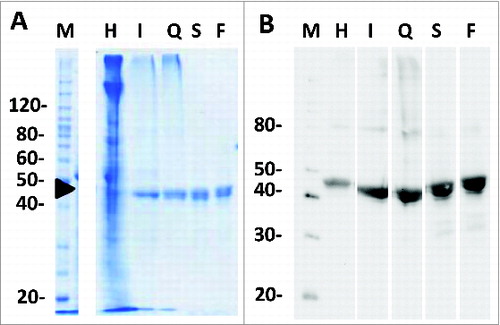
Figure 4. Representative elution profile for preparative SEC. Fractions include aggregate upslope (A1), aggregate downslope (A2), pre-monomer (M1), main monomer (M2) and monomer tail (MT). (B). SDS-PAGE (non-reducing, denatured) analysis of selected SEC fractions including A2, M1 with sub-fractions, and the leading edge of M2. The clearance of high molecular species eluting in A2 and M1 fractions was the impetus for limiting M2 as the main product peak.
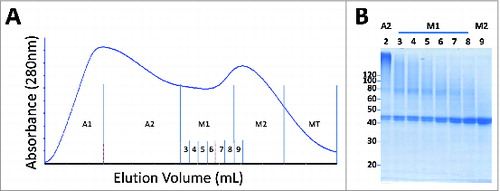
Figure 5. Analysis of the final purified Na-APR-1 (M74) protein on a 10% SDS-PAGE gel. Arrowhead indicates a 1.0 μg load. All samples were diluted with 1/5th volume 5x SDS loading dye and loaded in descending volumes from15 μL to 6 μL in 1 μL increments.
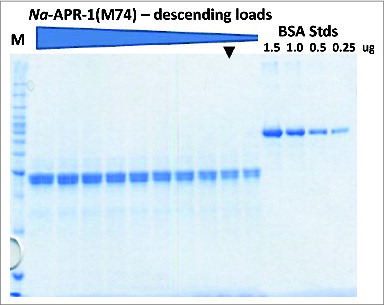
Figure 6. Analytical Fast Pressure Liquid Chromatography-SEC of the IMAC eluent, the anion exchange (AEX) eluent, and the final purified Na-APR-1 (M74) protein.
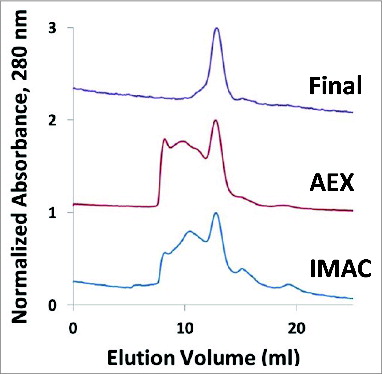
Table 1. Expression and solubility screening for Na-APR-1(M74) agrobacteria clone. Expression level and solubility of Na-APR-1 (M74) 5–7 days post infiltration (DPI) based on Western analysis
Figure 7. Evaluation of the purity profile of the Na-APR-1 (M74). (A): Analytical SEC HPLC chromatogram of the final purified Na-APR-1 (M74); (B): SDS-PAGE followed by Coomassie Blue Staining of Na-APR-1 (M74) and respective gel loading scheme (D), (C): Western Blot of Na-APR-1 (M74) probed with primary rabbit anti-WT-APR-1 antibodies and AP Goat Anti-Rabbit secondary antibodies and respective loading scheme (D); (D): Gel loading scheme for SDS-PAGE and Western Blot shown in (B and C).
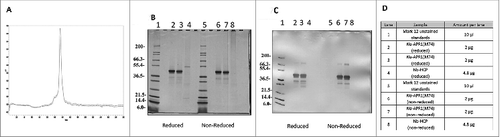
Figure 8. Electrtophoretic analysis of Na-APR-1 (M74) samples collected during accelerated stability study. Na-APR-1 (M74) was analyzed by SDS-PAGE followed by Coomassie Blue staining (left column), silver staining (center column) and Western Blot (right column) probed with anti-APR-1 antibodies. Na-APR-1 (M74) was incubated at 2–8°C, 25°C or 37°C for 7 (A, B, C), 15 (D, E, F) and 30 days (G, H, I). Gels were loaded as follows. Lane 1: Mark 12™ unstained standards, lane 2: Na-APR1 (M74) (reduced) 2–8°C, lane 3: Na-APR1 (M74) (reduced) 25°C; lane 4: Na-APR1 (M74) (reduced) 37°C, lane 5: Mark 12™ unstained standards, lane 6: Na-APR1 (M74) (non-reduced) 2–8°C, lane 7: Na-APR1 (M74) (non-reduced) 25°C; lane 8: Na-APR1 (M74) (non-reduced) 37°C.
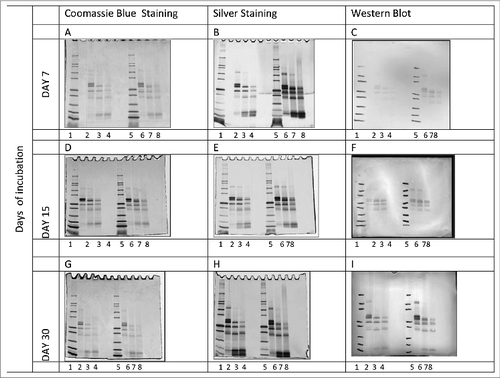
Figure 9. Analytical HPLC SEC of Na-APR-1 (M74) samples collected during accelerated stability studies. Samples were, incubated at 2–8°C, 25°C and 37°C for 7 (A, D, G), 15 (B, E,H) and 30 days (C, F, I).
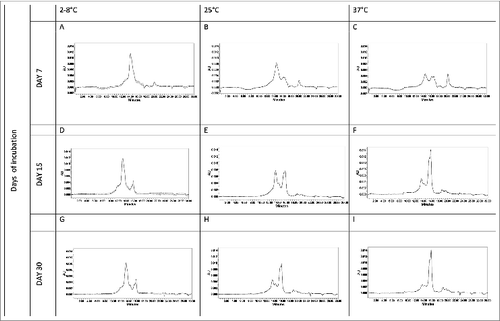
Table 2: A: Color and appearance, pH measurement and protein content by absorbance at 280nm of Na-APR-1 (M74) samples at day 0 and hold at 2–8°C, 25°C, and 37°C for 7, 15 and 30 days; B: Color and appearance, pH measurement and protein content by absorbance at 280nm of Na-APR-1 (M74) samples subjected to the 3 freeze/thaw cycles; C: SEC HPLC elution parameters of Na-APR-1 (M74) subjected to the 3 freeze/thaw cycles
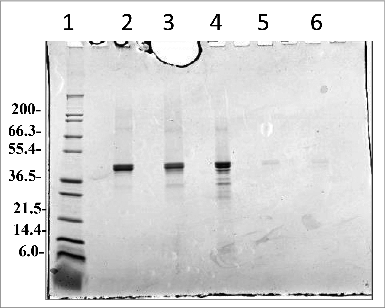
Figure 10. Freeze Thaw studies of Na-APR-1 (M74). A-C: Analytical HPLC SEC of Na-APR-1 (M74) after 3 (A, B, C) F/T cycles and reduced and non-reduced SDS-PAGE analysis followed by Coomassie Blue staining of the samples after 3 F/T cycles (D, Lane 2, and 4, respectively). Reduced and non-reduced western-blot analysis of the samples after 3 F/T cycles (E, lane 2 and 4, respectively), following the SDS-PAGE and western blot loading scheme defined in (E).
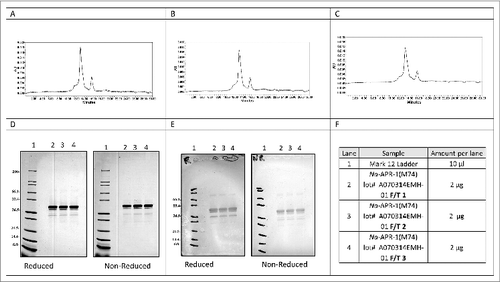
Figure 11. (A) Analytical HPLC SEC of Na-APR-1 (M74) after 24 month hold at −80°C and SDS-PAGE analysis followed by Coomassie Blue staining of Na-APR-1 (M74) after 24 months at −80°C. Lane 1 Mark12™ Marker, lane 2 and 3 Na-APR-1 (M74) non reduced, lanes 4 and 5 Na-APR-1 (M74) reduced.
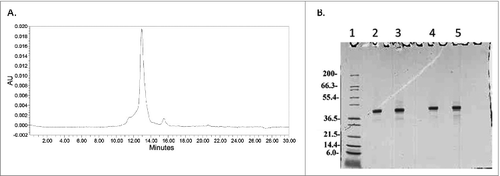
Figure 12. SDS-PAGE analysis followed by Coomassie Blue staining of the Na-APR-1 (M74) pellet and supernatant fractions after desorption from the Alhydrogel® adjuvant. After storage for 37 months at 2–8°C, the vaccine was treated with desorption buffer and separated into a pellet (lanes 2 and 3) and supernatant (lanes 5 and 6) fraction by centrifugation. Lane 1, Mark12™ molecular weight standards, lane 2 and lane 3 non-reduced pellet of Na-APR-1 (M74) after desorption, lane 4 non-reduced Na-APR-1 (M74) protein controls, lanes 5 and 6 non-reduced supernatant of Na-APR-1 (M74) once desorbed from the Alhydrogel®.