Figures & data
Figure 1. Effects of the movement against whole-cell pertussis vaccines on coverage and on disease epidemiology in the United Kingdom and Wales. Reprinted from The Lancet, Vol. 351, Gangarosa EJ, Galazka AM, Wolfe CR, Phillips LM, Gangarosa RE, Miller E, Chen RT, Impact of anti-vaccine movements on pertussis control: the untold story, pages 356–61, Copyright (1998), with permission from Elsevier.
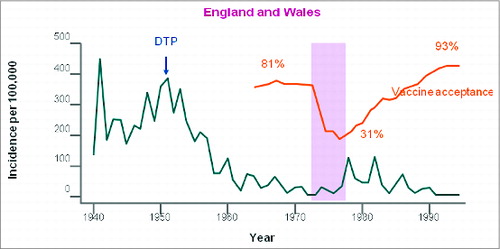
Figure 2. Factors guiding assessment of adjuvanted vaccine safety:Characteristics of the target population influence initial pre-clinicalevaluations. These features, as well as the results of pre-clinical testing, guide the specific assessment of vaccine safety in clinical trials.
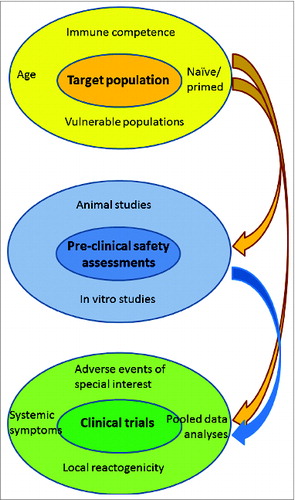
Figure 3. Post-licensure safety assessments are planned based on results of clinical trials, trends identified through spontaneous reports of adverse events after vaccination and field experience with the vaccine.
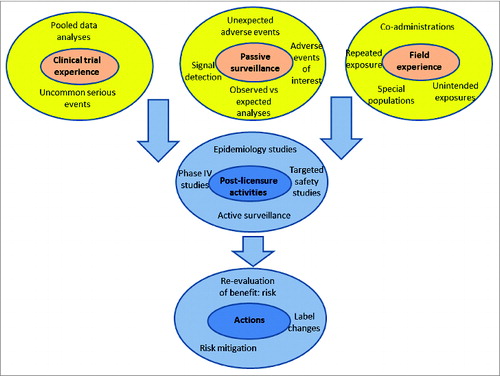
Figure 4. Factors potentially influencing the influence the benefit-risk profile of adjuvanted vaccines.
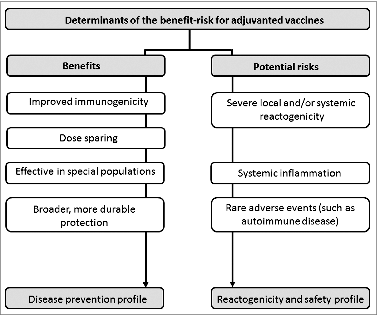
Table 1. Safety evaluation during the clinical development program
