Figures & data
Figure 1. The results of RT-PCR for the A549-TPS specificity band. M: molecular marker; Lane 1: amplicon of transfected control DCs; Lane 2: amplicon of A549; Lane 3: amplicon of immature DCs.
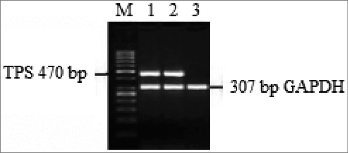
Figure 2. DCs were harvested and analyzed by FACS after labeling with the isotype controls and indicated mAbs. Fifty thousand cells were collected for each analysis. Immature DCs, ′transfected control′ DCs, ′gold standard′ DCs and ′improved′ DCs (with PGE2 or without PGE2) were analyzed with flow cytometry for various markers including CD11c (A), HLA-DR (B), CD80 (C), CD86 (D), CD83 (E), CXCR4 (F), CCR5 (G) and CCR7 (H). Percentages are expressed as mean ± SD of 3 independent experiments. Significant differences are shown (*P < 0.05, **P < 0.01, ***P < 0.001 ANOVA test); Brackets, at least P < 0.01; NS, p > 0.05.
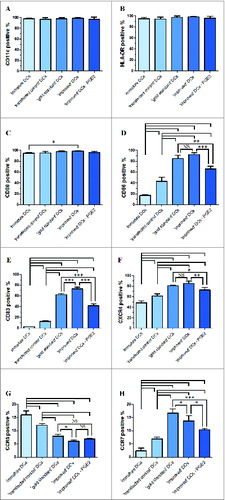
Figure 3. The levels of CCR7, CXCR4, CCL19, CCL21 and CXCL12 mRNA and β-actin (internal control) mRNA were determined by RT-PCR. After standardization to yield the same amount of β-actin mRNA, all samples mRNA levels were expressed as fold increase in respect to immature DCs (value = 1). (A) Chemokine receptor expression (CCR7 and CXCR4). (B) Chemokine expression (CCL19, CCL21 and CXCL12). All data represent the mean mRNA level ± SD obtained from 3 independent experiments. Significant differences are shown (*P < 0.05, **P < 0.01, ***P < 0.001 ANOVA test); Brackets, at least P < 0.01; NS, p > 0.05.
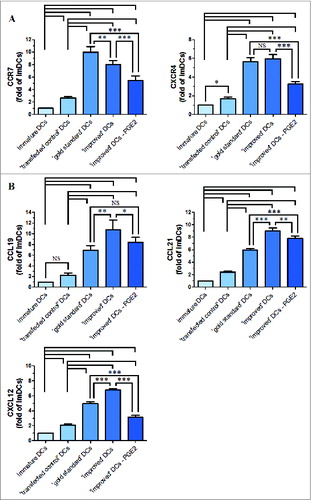
Figure 4. (A) A representative western blot analysis of CCR7 and CXCR4 protein expression levers in immature DCs and matured DCs is shown. Levels of β-actin were determined and served as loading control. Lane 1, immature DCs; Lane 2, ′transfected control′ DCs; Lane 3, ′gold standard′ DCs; Lane 4, ′improved′ DCs; Lane 5, removal of PGE2 from the ′improved′ DCs. (B) Western blot analysis of CCR7 and CXCR4 protein expression in immature and DCs matured by cytokine cocktail is shown. All results obtained were from 3 independent experiments and are presented as means ±SD. NS, p > 0.05.
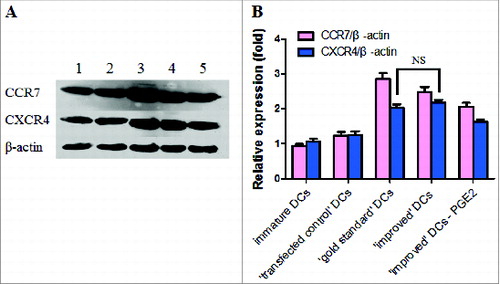
Figure 5. Inflammatory cytokine secreted by immature or maturing DCs in response to various maturation stimuli and the production of IL-12p70 (A) and IL-10 (B) was determined in the culture supernatants. Data in bars are expressed as means of cytokine concentrations ± SD of 3 independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001 ANOVA test); ⊥ indicates ′or′.
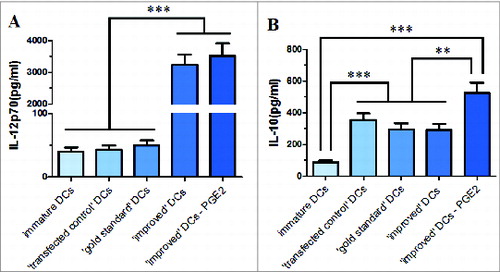
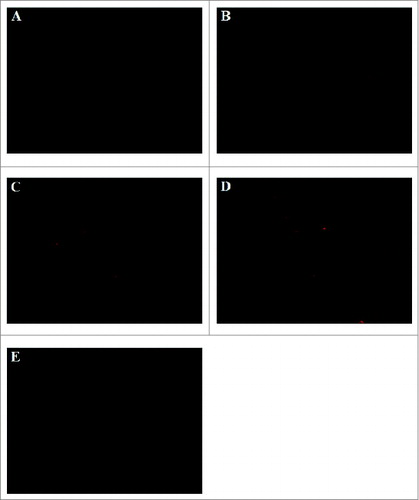
Figure 6. Spleen histological cryosections staining red fluorescence of CM DiI-labeled DCs was detected and images were acquired with Olympus IX51 (Tokyo, Japan) fluorescence microscope using a 100 × objectives. CM DiI-labeled DCs mainly located in periarterial lymphatic sheath and marginal zone. (A) ′immature′ DCs (B) ′transfected control′ DCs; (C) ′gold standard′ DCs; (D) ′improved′ DCs; (E) removal of PGE2 from the ′improved′ DCs.