Figures & data
Figure 1. For figure legend, see page 1692. Figure 1 (See previous page). Study design (A) and flow of participants through the trial to month 12 (B). Syringe symbols represent vaccine administration. AE, adverse event; ATP-I, according to protocol immunogenicity cohort; BS (1), blood sample for assessment of antibodies (by enzyme-linked immunosorbent assay in all subjects and by pseudovirion-based neutralisation assay in a subset of subjects); BS (2), blood sample for assessment of cell-mediated immunity in a subcohort of subjects; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine at months 0 and 6; HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine at months 0 and 6; HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine at months, 0, 2 and 6; M, month; TVC, total vaccinated cohort. *number present in one group only, but replicated to avoid unblinding.
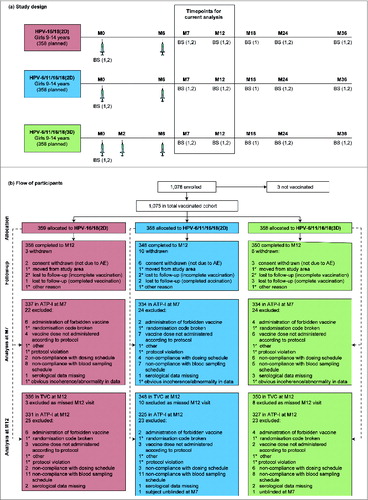
Table 1. Demographic characteristics and baseline serostatus in the TVC and ATP-I (at one (M7) and 6 months (M12) after the last vaccine dose)
Figure 2. HPV-16-specific humoral and cellular immune responses for initially seronegative subjects in the ATP-I at one (M7) and 6 months (M12) after the last vaccine dose. For panels (A and B), bars show geometric mean antibody titers (GMTs) and associated 95% confidence intervals; the numbers within each bar are the GMTs for each group. For panels (C and D), boxplots show median, lower quartile, upper quartile, minimum and maximum frequency of cells; the numbers within each bar are the median values for each group. ATP-I, Month 12 according-to-protocol immunogenicity cohort; ED50, effective dose producing 50% response; ELISA, enzyme-linked immunosorbent assay; EU/mL, ELISA units per milliliter; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine at months 0 and 6; HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine at months 0 and 6; HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine at months, 0, 2 and 6; M, month; n, number of subjects with available results; Nat. inf., historical GMTs for women who had cleared a natural infection (29.8 EU/mL for ELISA; 180.1 ED50 for PBNA);Citation1,20 PBNA, pseudovirion-based neutralisation assay; Plateau, historical GMT (ELISA) at months 45–50 for women aged 15–25 y participating in a long-term efficacy trial (397.8 EU/mL).Citation8 The cut-off values for the ELISA and PBNA assays were 19 EU/mL and 40 ED50, respectively.
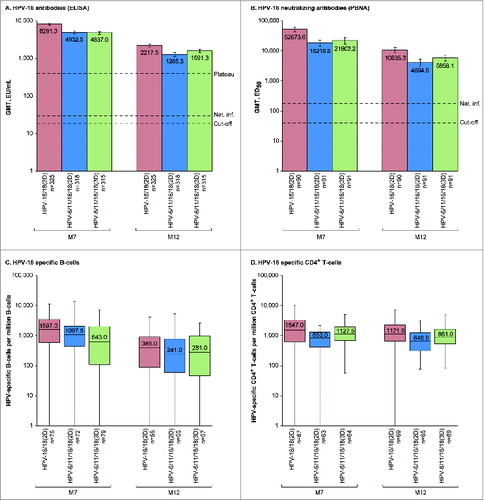
Figure 3. HPV-18-specific humoral and cellular immune responses for initially seronegative subjects in the ATP-I at one (M7) and 6 months (M12) after the last vaccine dose. For panels (A and B), bars show geometric mean antibody titers (GMTs) and associated 95% confidence intervals; the numbers within each bar are the GMTs for each group. For panels (C and D), boxplots show median, lower quartile, upper quartile, minimum and maximum frequency of cells; the numbers within each bar are the median values for each group. ATP-I, Month 12 according-to-protocol immunogenicity cohort; ED50, effective dose producing 50% response; ELISA, enzyme-linked immunosorbent assay; EU/mL, ELISA units per milliliter; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine administered at months 0 and 6; HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine administered at months 0 and 6; HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine administered at months, 0, 2 and 6; M, month; n, number of subjects with available results; Nat. inf., historical GMTs for women who had cleared a natural infection (22.6 EU/mL for ELISA; 137.3 ED50 for PBNA);Citation1,20 PBNA, pseudovirion-based neutralisation assay; Plateau, historical GMT (ELISA) at months 45–50 for women aged 15–25 y participating in a long-term efficacy trial (297.3 EU/mL).Citation8 The cut-off values for the ELISA and PBNA assays were 18 EU/mL and 40 ED50, respectively.
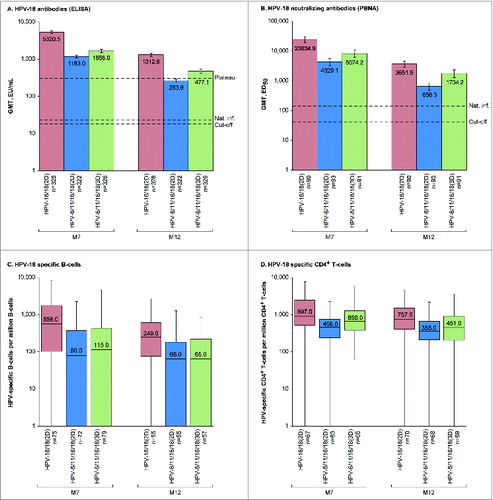
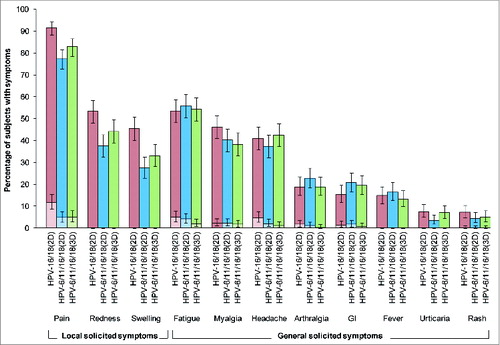
Figure 4. Incidence of local and general solicited symptoms during the 7-day post-vaccination period overall per subject for the total vaccinated cohort. For each group, the darker and lighter shaded bars, respectively, represent the percentage of subjects with any symptom or any grade 3 symptom with exact 95% confidence intervals. GI, gastrointestinal; HPV-16/18(2D), 2 doses of the HPV-16/18 AS04-adjuvanted vaccine administered at months 0 and 6 (n = 359); HPV-6/11/16/18(2D), 2 doses of the HPV-6/11/16/18 vaccine administered at months 0 and 6 (n = 357); HPV-6/11/16/18(3D), 3 doses of the HPV-6/11/16/18 vaccine administered at months, 0, 2 and 6 (n = 356); girls in the 2D groups received placebo at dose 2. Fever was defined as oral or axillary temperature ≥37.5°C; grade 3 fever was defined as oral or axillary temperature >39.0°C. Grade 3 redness/swelling were defined as an area at the local injection site with diameter >50 mm. For all other symptoms, a grade 3 event was defined as one which prevented normal everyday activities.