Figures & data
Figure 1. TEM image of Enterovirus 71 (EV71) virus-like particles. The morphology of purified VLPs via affinity chromatography was characterized by negative staining under TEM. Scale bar = 100 nm.
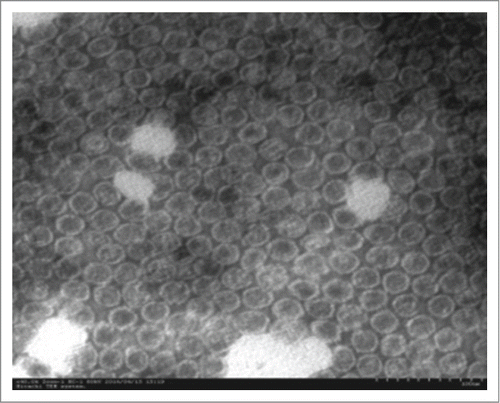
Figure 2. Neutralization titer of the different dosage of EV71 VLPs serum was measured by the micro-neutralization assay. The VLP immune serum samples at dilutions from 23 to 214 were mixed with 100 TCID50 of EV71 viruses and incubated with RD cells at 35°C for 7 d The neutralization antibody titer was defined as the highest serum dilution that prevented the occurrence of cytopathic effects.
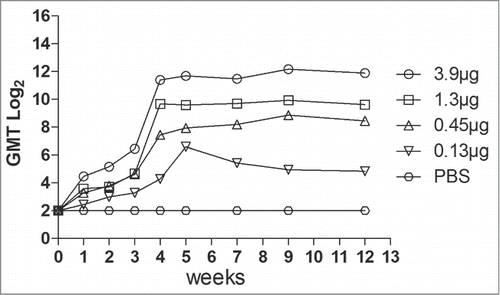
Figure 3. Antigen-specific IgG titers in the serum samples of mice immunized with different doses of the EV71 VLPs. Results are presented as the mean ± SD of 3 independent experiments.
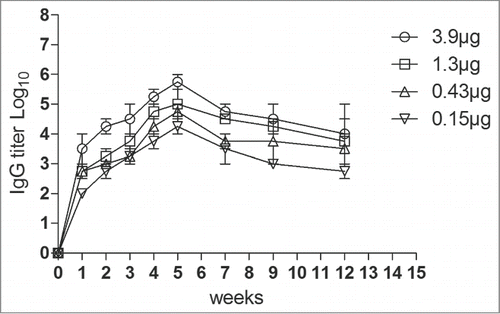
Figure 4. Maternal antibody protected the newborn mice against EV71 lethal challenge. Female ICR mice (6–8 week) were i.p. injected with different dosage vaccines and PBS at a 3-week interval and allowed to mate at 1 week after the first injection. After 3 weeks, the newborn mice were challenged with 15 LD50 of the EV71 challenge virus. Mortality and clinical disease were monitored and recorded daily after infection. The Mantel-Cox log-rank test was used to compare the survival of pups between each maternal immunization group and the PBS control group at 21 d post-infection.
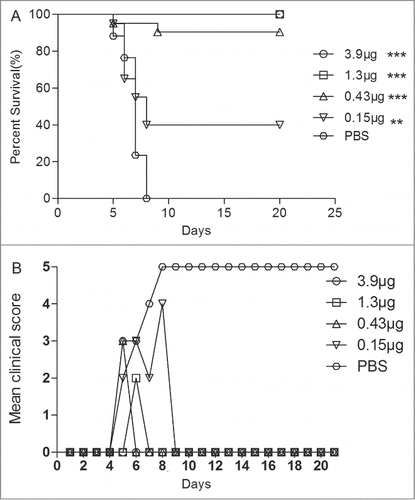
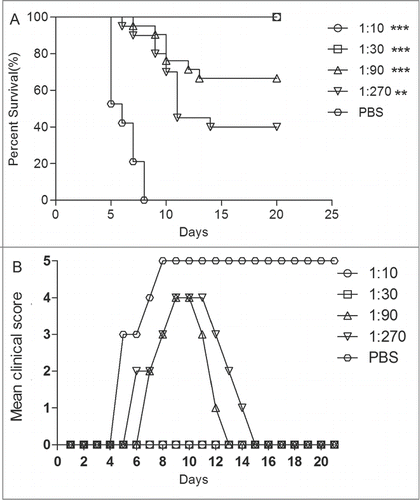
Figure 5. The newborn mice protection with anti-EV71 VLPs serum against EV71 lethal challenge in vivo. One-day-old ICR mice (n = 8–10 per group) were i.p. inoculated with 30 μl of the fold3- serially diluted anti-EV71 serum (NTAb titer = 4096) or PBS serum (NTAb titer <8). Within 1 h post-inoculation, each mouse was i.c. injected with 15LD50 of the EV71 challenge virus. Mortality and clinical disease were monitored and recorded daily after infection. The Mantel-Cox log-rank test was used to compare the survival of pups between each antiserum group and the PBS control group at 21 d post-infection. ***, P < 0.0001; **, P < 0.001.
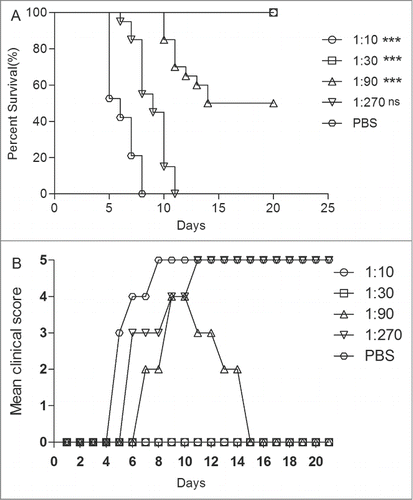
Figure 6. The newborn mice protection with pretreated anti-EV71 VLPs serum against EV71 lethal challenge in vivo. Serially diluted anti-EV71 serum (NTAb titer 4096) or PBS serum (NTAb titer <8 ) was incubated with 15LD50 of the EV71 challenge virus at 37°C for 1 h. One-day-old ICR mice (n = 8–10 per group) were i.c. injected with the serum-virus mixture. Mortality and clinical disease were monitored and recorded daily after infection. The Mantel-Cox log-rank test was used to compare the survival of pups between each antiserum group and the PBS control group at 21 d post-infection. ***, P < 0.0001; **, P < 0.001.