Figures & data
Figure 1. Schematic presentation of 2V(5–1) fractional factorial design of experiments for immunogenicity assay optimization. (A) Specification of operating factors and their levels. (B) Specification of experimental plan; experimental runs with invalid results (bellow the detection level of the assay) that were not included in the data analysis are denoted in gray.
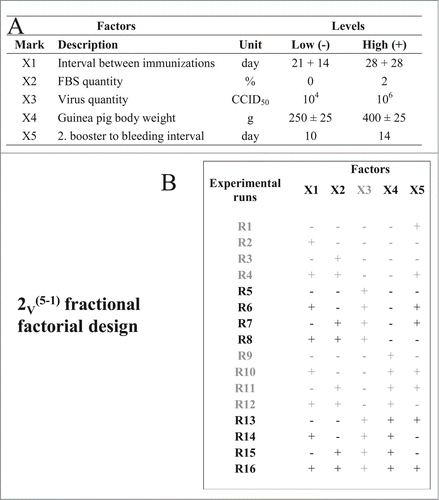
Figure 2. Specification of valid experiments recognized as 2IV(4–1) fractional factorial design (A) and immunogenicity assay results measured by virus neutralization assay (B) and HI assay (C). Results are expressed as mean (◊) and 95% confidence interval (CI) (⊥) of logarithmic values of individual results within each run. Full line denotes the mean and dashed lines denote 95% CI of the logarithmic values of total experimental plan, calculated from all individual values.
Figure 3. Data analysis of immunogenicity assay optimization according to 2IV(4-1) experimental design. (A) Mean value (◊) of immunogenicity assay outcome at higher (+1; ) and lower (−1;
) level of each experimental factor (X1 - intervals between immunizations; X2 – FBS quantity; X4 – guinea pig body weight; X5 – interval between 2. booster and bleeding) in comparison to mean value (full line) and 95% confidence interval (dashed lines) of the total experimental plan. Immunogenicity assay outcome was measured by virus neutralization assay (upper graphs) and HI assay (lower graphs). (B) Pareto plot of main effect estimates, presented by horizontal columns. The statistically significant effects (p = 0.05) are denoted by *. Immunogenicity assay outcome measured by virus neutralization assay (left) or HI assay (right).
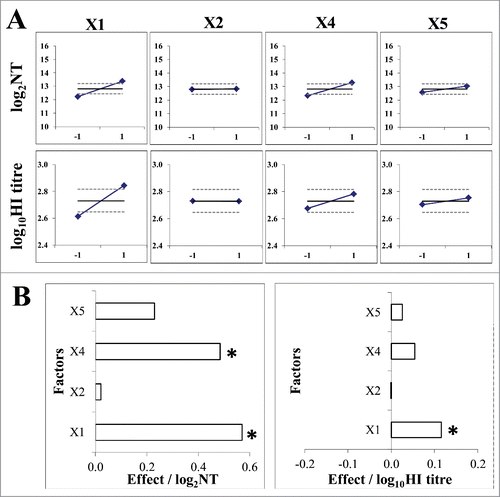
Figure 4. Virus quantity in immunization dose / immunogenicity assay outcome relationship. Mean values (◊) and 95% confidence interval (⊥) of the MuV-specific antibody titer (expressed in log10HI titer) obtained in each experimental group (n = 5) were plotted against the MuV quantities in the immunization dose. Linear relationship was obtained in the range 5.1 to 6.3 logCCID50 (125,000 to 1,000,000 CCID50) per dose.
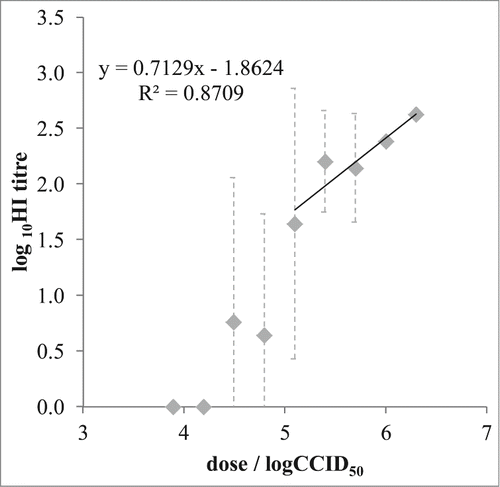
Figure 5. Influence of FBS in the immunization sample on the immunogenicity of the MuV. Groups of 5 animals were immunized with 500,000 CCID50 of LZG MuV per immunization dose, either without FBS in the sample (MuV) or with 2% FBS (MuV-FBS I and MuV-FBS II), prepared as follows. The unique sample of MuV was split in 2 aliquots, and one completed with 2% FBS (MuV-FBS sample), the other with equal volume of PBS (MuV sample) prior storage. MuV-FBS sample (virus titer 8.566 ± 0.226 log CCID50) was diluted with 2% FBS and suspension was used for immunization of a group named MuV-FBS I. MuV sample (virus titer 8.208 ± 0.373 logCCID50) was diluted either with pure PBS to obtain immunization suspension for the group MuV, or with 2% FBS for the immunization of the group MuV-FBS II. Medians (◊) with minimal and maximal values (⊥) of the MuV-specific antibody titer (expressed in log10HI titer) obtained in each experimental group (n = 5) are given; *p < 0.05 in comparison to MuV group.
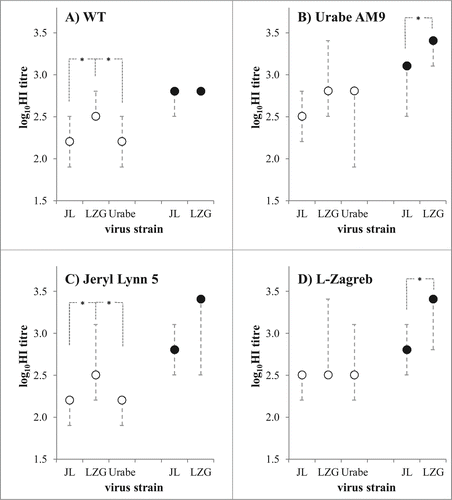
Figure 6. Comparison of 3 mumps vaccine strains in guinea pig immunogenicity assay. MuV specific antibody quantities were measured by 4 HI assays, differing in virus that was used as a hemagglutinating agent: (A) wild type virus MuVi/Split.CRO/05.11 strain (denoted as WT); (B) Urabe AM9; (C) Jeryl Lynn 5; and (D) L-Zagreb. Animals (n = 5 per group) were immunized with the same quantity (6.47 logCCID50 per dose) of each MuV strain (denoted on x-axis as LZG for L-Zagreb, Urabe for Urabe AM9 and JL5 for Jeryl Lynn 5). All three viruses were tested when FBS was included in immunization suspension (empty circles), and 2 of them were tested also without FBS in immunization suspension (full circles). Medians with minimal and maximal values of log10HI titre are given; *p < 0.05 between groups connected with dashed lines.