Figures & data
Figure 1. Flow of participants through the trial. 2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, licensed HPV-16/18 AS04-adjuvanted vaccine formulation containing 20 μg each of HPV-16 and −18 L1 virus-like particles and adjuvanted with AS04; 40/40, alternative HPV-16/18 AS04-adjuvanted vaccine formulation containing 40 μg each of HPV-16 and −18 L1 virus-like particles and adjuvanted with AS04; ATP-I, according-to-protocol immunogenicity cohort; M, month; y, years. *Excluding one subject who attended the Month 60 visit but did not sign the informed consent form for this visit. This article focuses on subjects randomized to receive the HPV-16/18 AS04-adjuvanted licensed vaccine formulation (3D 20/20 M0,1,6 and 2D 20/20 M0,6 groups; shaded boxes). Disposition data are also shown for subjects randomized to receive the alternative HPV-16/18 AS04-adjuvanted vaccine formulation (2D 40/40 M0,6 and 2D 40/40 M0,2 groups) for completeness.
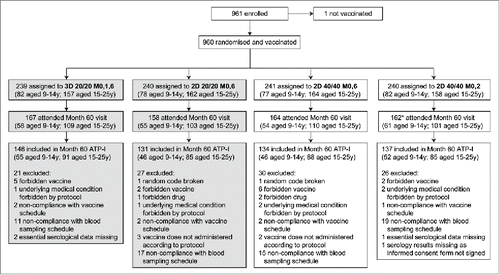
Table 1. Summary of demographic characteristics and baseline serostatus by age stratum
Figure 2. Observed HPV-16 and −18 geometric mean antibody titers (GMT) and corresponding 95% confidence intervals (CI) by enzyme-linked immunosorbent assay (ELISA) at each time point for subjects in the Month 60 according-to-protocol immunogenicity cohort (ATP-I) who were seronegative at baseline for the corresponding antigen. 2D, 2-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation; 3D, 3-dose schedule of the licensed HPV-16/18 AS04-adjuvanted vaccine formulation. M, month; N, number of evaluable seronegative subjects in the Month 60 ATP-I; Plateau, GMTs at the plateau level (Month 45-50 time point) in women aged 15-25 y administered 3 doses of the HPV-16/18 AS04-adjuvanted vaccine at months 0, 1 and 6 in a previous trial (NCT00120848) were 397.8 and 297.3 ELISA unit (EU)/mL for HPV-16 and −18 antibodies, repectively.Citation16 Natural infection, GMTs in women aged 15-25 y who had cleared a natural infection in a previous trial (NCT00122681) were 29.8 and 22.7 EU/mL for HPV-16 and −18 antibodies, repectively.Citation1
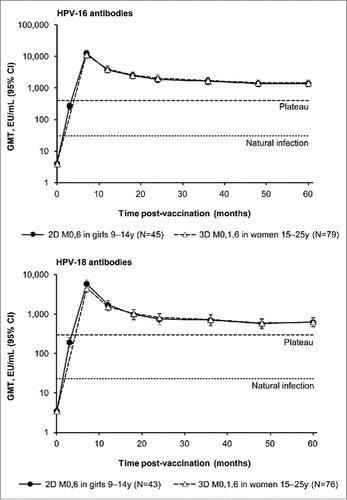
Table 2. Observed HPV-16 and −18 antibody responses by ELISA at Month 60 for initially seronegative subjects in the ATP-I
Figure 3. Predicted HPV-16 and −18 geometric antibody mean antibody titers (GMTs) over 20 y, as predicted by the piecewise model (panels A and B) and modified power law model (panels C and D). Blue solid line, predicted GMTs for girls aged 9-14 y administered 2 doses of the licensed HPV-16/18 AS04-adjuvanted vaccine at months 0 and 6, modeled on the basis of 5 y of follow-up data from the current study. Red dashed line, predicted GMTs for women aged 15-25 y administered 3 doses of the licensed HPV-16/18 AS04-adjuvanted vaccine at months 0, 1 and 6, modeled on the basis of 5 y of follow-up data from the current study. Green dotted line, predicted GMTs for women aged 15-25 y administered 3 doses of the licensed HPV-16/18 AS04-adjuvanted vaccine at months 0, 1 and 6, modeled on the basis of 6.4 y of follow-up data from a previous efficacy study (NCT00120848).Citation21 Black dashed line, GMTs in women who had cleared a natural infection in a previous trial (NCT00122681) (29.8 and 22.7 ELISA unit (EU)/mL for anti-HPV-16 and −18, respectively).Citation1 ELISA, enzyme-linked immunosorbent assay.
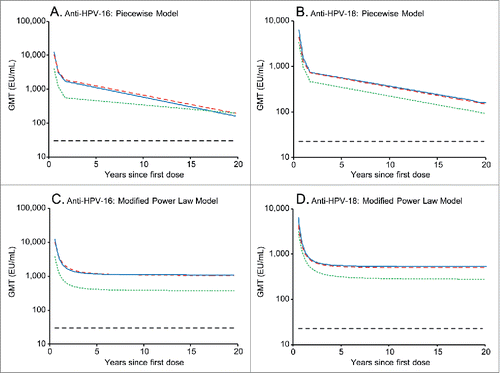
Table 3. Predicted HPV-16 and −18 antibody titers after 20 y and duration of persistence
Table 4. Summary of safety and pregnancy outcomes in the total vaccinated cohort (TVC)
