Figures & data
Figure 1. Vaccine antigens expressed in vitro. Each lane was loaded with the lysed proteins from transfected products. From left to right: blank control, p4.0-Gag, p4.0-hCyPA, p4.0-Gag + p4.0-hCyPA, p4.0-Gag/hCyPA, p4.0-Gag/hCyPA-3mut, p4.0-Gag/hCyPA-H54Q, p4.0-Gag/hCyPA-F60A, and p4.0-Gag/EGFP. All plasmids were transfected into 293T cells separately, and cells were harvested 48 h later for western blot analysis. Anti-Gag, anti-CyPA, and anti-β-actin antibodies were used to test each protein expression. Results are representative of 2 independent experiments.
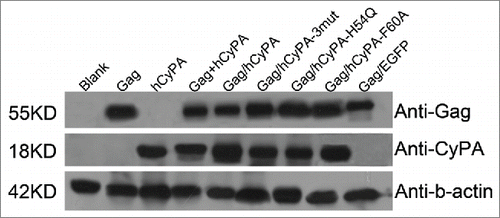
Figure 2. Assessment of CyPA species-specificity and antigen-specificity of the adjuvant effect. The Gag-specific cellular immune response was assessed by IFN-γ ELISPOT array to investigate the difference between the mouse and human CyPA adjuvant effect when co-administrated with Gag vaccine (A). Ten 6- to 8-week-old female Balb/c mice in each group were administered vaccines under Scheme I in , with CyPA plasmid mixed with Gag vaccine before immunization, and a 50 μg DNA vaccine at a final volume of 100 μl each (50 μl for each tibialis anterior muscle) injected directly. Mice were immunized 3 times at 2-week intervals with Gag DNA plasmid co-formulated with CyPA adjuvant DNA. One week after the last vaccination, animals were sacrificed, and individual mouse splenocytes were isolated and used to assess cellular function by IFN-γ ELISPOT. The CyPA-Gag antigen specific adjuvant activation (B) was tested following Scheme II. We designed a multiplex comparison to verify the CyPA adjuvant effect on Gag antigen specificity. “Gag + hCyPA sep” denotes Gag and hCyPA plasmid injected separately into the tibialis anterior muscles of 2 legs. Moreover, Env DNA, an HIV antigen, was tested for specificity of CyPA adjuvant activation. All the procedures of immunization, timeline, interval time and operation were followed method and material session description. The p values are labeled with asterisks for p < 0.05 (*) and p < 0.0001 (****). In addition, N.S indicates the non-significant difference between the compared groups.
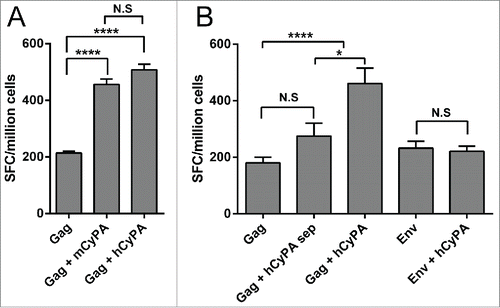
Table 1. The mouse immunization strategy
Figure 3. Gag/CyPA dual expression cassette strategy augments Gag cellular immunity and the CyPA mutant effect on adjuvant activation. To investigate the efficiency of the Gag/CyPA dual expression cassette vaccine and identify the mechanism of CyPA adjuvantivity on the Gag vaccine with CyPA mutants, the Gag-specific cellular immune response was assessed by IFN-γ ELISPOT array. Ten 6- to 8-week-old female Balb/c mice in each group were vaccinate according to Scheme III in . p4.0-Gag/hCyPA (68 μg) was used instead of a 50 μg dose to keep the same Gag gene copy number in the different vaccines, and was compared with 50 μg of p4.0-Gag DNA to assess the immunogenicity of the Gag/CyPA chimeric vaccine. Multi-site mutations of H54Q/R55A/F60A CyPA markedly induced a Gag-specific cellular immune response. To explore this finding, we determined the effect of 2 single-site CyPA mutants (H54Q and F60A) in a DNA vaccine. All mice were immunized 3 times at a 2-week intervals, and sacrificed one week after the last vaccination. The IFN-γ ELISPOT array was used to investigate the antigen-specific cellular immune response induced by the vaccination regimens. The p values were labeled with asterisks for p < 0.05 (*), p < 0.01(**), p < 0.001 (***), and p < 0.0001 (****).
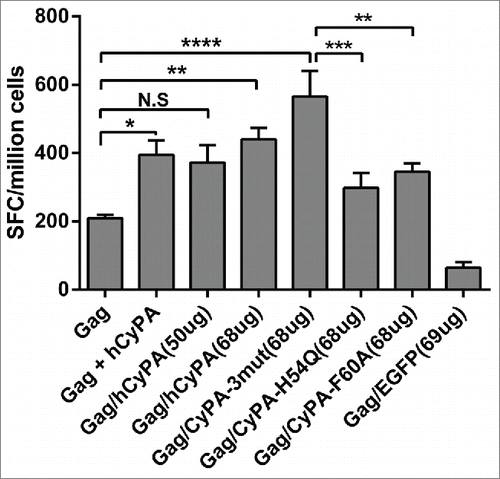
Figure 4. Identification of dominant Gag epitopes in various vaccination strategies by ELISPOT mapping assay. The figure indicates the T-cell responses of splenocytes from immunized mice to 22 pools, each consisting of 11 Gag 15-aa peptides overlapping by 11-aa, that were measured by ELISPOT assay 2 weeks after the last of 3 DNA immunizations. The black box highlights pools (p value < 0.05) that induced significantly greater cellular immune responses in the CyPA groups, compared with the Gag-immunized only group. The dashed line at 20 spots indicates the cut-off value of this assay.
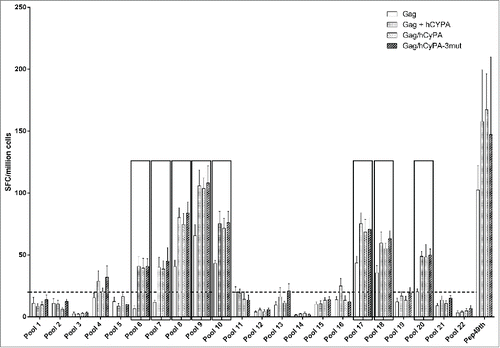
Figure 5. Individual epitopes identified by ELISPOT mapping following distinct immunization regimens. To narrow down the dominant epitopes determined by pool mapping, several individual peptides were selected through cross-referencing of Gag peptides mapped in the pool matrix (Table S1). The IFN-γ ELISPOT assay was performed to verify the efficiency of each single peptide in inducing Gag-specific cellular immunity. The asterisk denotes significant differences (* indicates p < 0.05 and ** indicates p < 0.01) compared with the Gag only group.
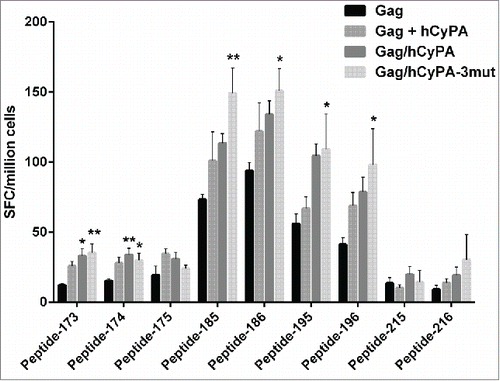
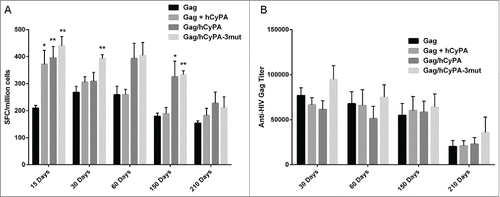
Figure 6. Long-term immunity monitored up to 7 months after the last vaccination. Long-term cellular immune response detected by IFN-γ ELISPOT assay (A). We monitored the dynamic changes of vaccine-induced Gag cellular immunity at 15, 30, 60, 150, and 210 days after the last immunization. The asterisks indicate significant differences, with p < 0.05 (*) and p < 0.01(**), in comparison with the Gag only group at the same time point. Binding antibody titer analyzed by ELISA (B). The dynamic changes in vaccine-induced Gag-specific binding antibody were analyzed at 30, 60, 150, and 210 days after the last immunization in each vaccine group.