Figures & data
Figure 1. Flowchart illustrating the study population and experimental design. The study was based on two branches: The humoral (A) and cellular (B) immunology analysis. The eligible population comprises 171 adults. Blood collections (without anticoagulant for humoral analysis and in heparinized tubes for cellular immunology assessment) were performed prior vaccination (NVday0 (n=39) and at different timepoints after primary vaccination: PVday30-45 (n=39); PVyear1-4 (n=36); PVyear5-9 (n=12); PVyear10-11 (n=45) and PVyear12-13 (n=39).
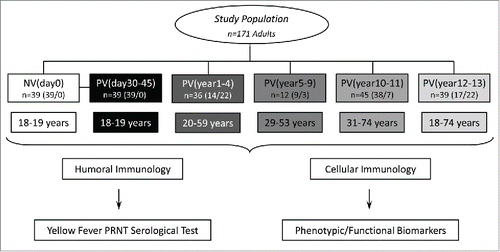
Figure 2. Immunogenicity following 17DD-YF primary vaccination. (A) Anti-YF neutralizing antibody titers were measured by PRNT carried out before NVday0 (n=39) and at different time-points after primary vaccination: PVday30-45 (n = 39); PVyear1-4 (n = 36); PVyear5-9 (n = 12); PVyear10-11 (n = 45) and PVyear12-13 (n = 39). PRNT antibody titers are expressed in log10 mIU/mL. (B) Seropositivity rates were determined by the PRNT value of 2.9 log10 mIU/mL as the cut-off point to segregate seropositive from seronegative samples and data analysis performed by multivariate logistic regression analysis modeled as a function of the time (in months) elapsed since vaccination as categories. (C) Correlation analysis of Anti-YF neutralizing antibody titers after primary vaccination at different time-points using linear regression fit curve. Spearman's correlation r index and p values are displayed in the lower corner of the graph. Significant differences at p < 0.05 as compared to NVday0 time-point are displayed as “a” and differences as compared to PVday30-45 are displayed as “b”.
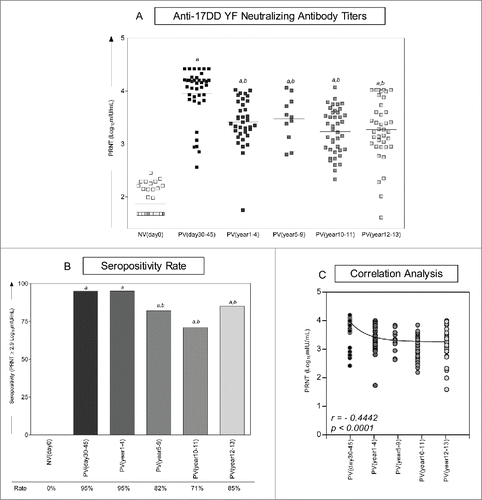
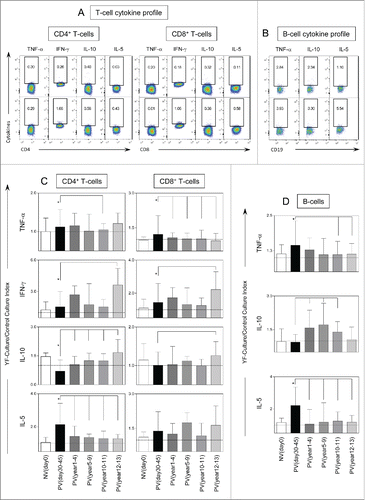
Figure 3. Timeline of memory phenotypic features following 17DD-YF primary vaccination. (A) Flow cytometric dot plots representing the memory T-cell phenotypes and (B) memory B-cell phenotypes. (C) memory T-cell phenotypes such as naïve, early effector, central and effector memory T-cells as well as (D) B-cell phenotypes such as naïve, nonclassical and classical memory are represented by YF-Culture/Control culture index plotted as bar graphs for healthy adults prior vaccination NVday0 (n=39) and at different time-points after primary vaccination: PVday30-45 (n = 39); PVyear1-4 (n = 36); PVyear5-9 (n = 12); PVyear10-11 (n = 45) and PVyear12-13 (n = 39). Significant differences at p < 0.05 as compared to NVday0 time-point are displayed as “*” and differences as compared to PVday30-45 time-point are displayed as connecting lines.
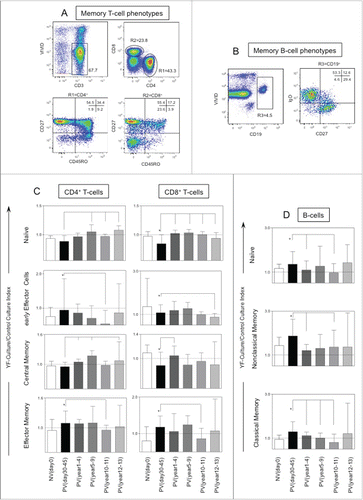
Figure 5. Phenotypic and functional memory analysis following 17DD-YF primary vaccination along time. Radar graphs represent the frequency of high producers of memory and functional phenotypic subsets relevant to assessing the immune response before and different times following primary vaccination. Memory phenotypic features were plotted on the left half, while the functional cytokine-producing T and B-cells were plotted at the right half of each radar graph. The inner circle represents the 50th percentile for each parameter, which was taken as threshold to define relevant frequency of subjects with higher levels of a given biomarker (*). Circles in gray, black and white refer to CD4+, CD8+ T-cells and B-cells, respectively. N – naïve, eEF – early effector, CM – Central Memory and EM – Effector Memory.
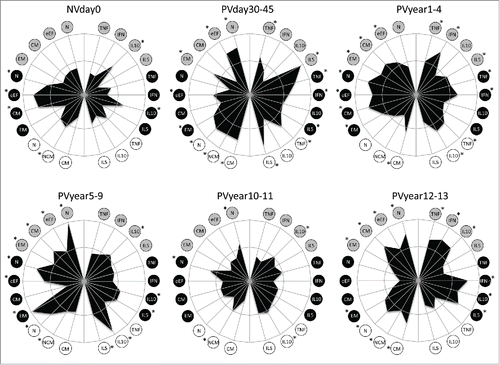
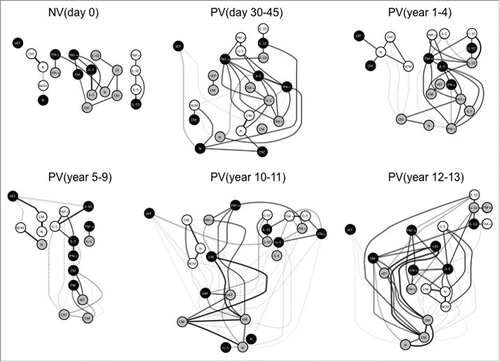
Figure 6. Systems biology analysis of 17DD-YF primary vaccination over time. Networks were assembled by assessing the association memory and functional phenotypic subsets relevant to assessing the immune response after 17DD-YF primary vaccination at each time-point. Only significant positive and negative correlations (p < 0.05) are displayed. Continuous lines represent direct or positive correlations, whereas dotted lines represent inverse or negative correlations defined by spearman's correlation r index, as described in material and methods. The correlation index (r) defined the strength of association as moderate when 0.36 > r < 0.67 (thin lines) or strong if r > 0.68 (thick lines). Circles in gray, black and white refer to CD4+, CD8+ T-cells and B-cells, respectively. N – naïve, eEF – early effector, CM – Central Memory and EM – Effector Memory.