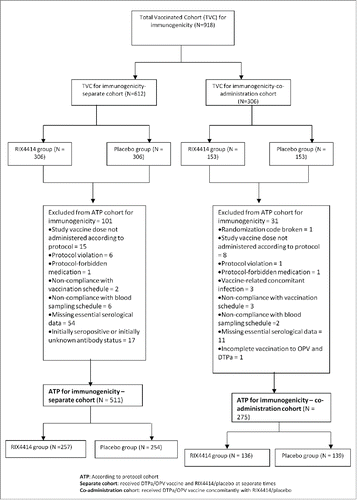
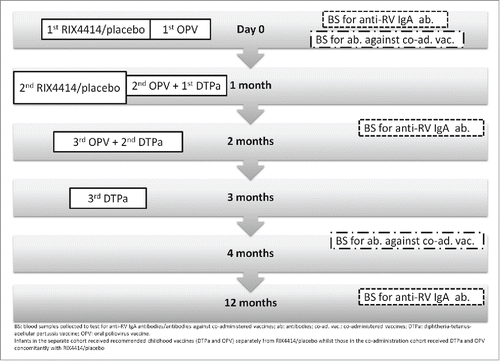
Figures & data
Table 1. Demographic characteristics (ATP immunogenicity cohort).
Figure 2. Anti-RV IgA seroconversion (one month post-dose-2)/seropositivity rates (at one year of age) and GMCs in seropositive subjects (separate and co-administration cohort [vaccine group]) (ATP- cohort): (a) Seroconversion / Seropositivity rates; (b) GMCs.
![Figure 2. Anti-RV IgA seroconversion (one month post-dose-2)/seropositivity rates (at one year of age) and GMCs in seropositive subjects (separate and co-administration cohort [vaccine group]) (ATP- cohort): (a) Seroconversion / Seropositivity rates; (b) GMCs.](/cms/asset/2e7e388f-67bb-42f7-ab63-e25266030e20/khvi_a_1085143_f0002_b.gif)
Table 2. Immune responses toward co-administered vaccines one month after the third vaccine dose (co-administration cohort).
Table 3. Solicited symptoms (any symptom and general symptoms).