Figures & data
Figure 1. Expression of LdNH36 constructs in P. pastoris X-33 compared with E. coli-expressed wild-type LdNH36, evaluated by Western blot with anti-LdNH36 mouse sera (reduced 4–20% Tris-glycine gel/chemiluminescence detection). Lane 1: 10 µL of wild-type LdNH36 culture supernatant expressed in P. pastoris; Lane 2: 10 µL of LdNH36-dg culture supernatant expressed in P. pastoris; Lane 3: 10 µL of LdNH36-dg2 culture supernatant expressed in P. pastoris; Lane 4: 50 ng (determined by 280 nm absorbance) of purified, His-tagged wild-type LdNH36 expressed in E. coli as control.
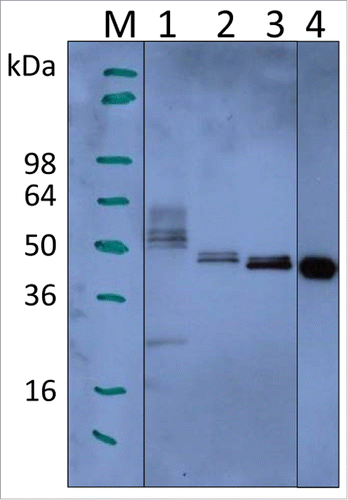
Figure 2. Amino acid sequence of LdNH36-dg2. The 4 N-glycosylation site mutations (N!Q) are underlined. None of the mutation sites are present in F3 region (bold), which showed highest protection in studies by Nico et al., 2010.

Figure 4. Fermentation results with reduced 4–20% Tris-glycine gels. (A) SDS-PAGE with Coomassie Blue staining and (B) Western blot with anti-LdNH36/colorimetric detection. Lane M: molecular weight marker; Lane 1: pre-induction; Lane 2: 72 h of induction; Lane 3: 96 h of induction.
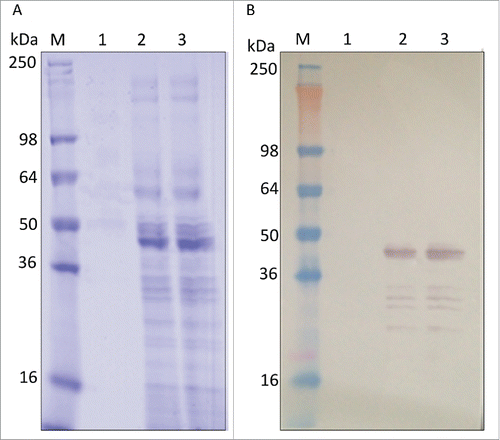
Figure 5. In-process sample analysis with 4–12% Bis-Tris gels. (A) SDS-PAGE with Coomassie blue staining, (B) Western blot with anti-LdNH36/colorimetric detection), and (C) HCP Western blot with anti-P. pastoris/colorimetric detection. Lanes 1–6 are non-reduced and lanes 7–8 are reduced. Lanes M: molecular weight standard; Lane 1: fermentation supernatant; Lane 2: post-TFF; Lane 3: Capto SP pool; Lane 4: concentrated Capto SP pool; Lane 5 and 8: SEC200 pool low load; Lane 6 and 7: SEC200 pool high load.
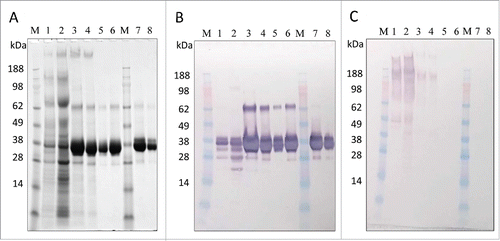
Table 1. Purification table from 20 L run SDS-PAGE densitometry.
Figure 6. HPLC-SEC and DLS characterization of LdNH36-dg2. (A) HPLC-SEC chromatographs of in-process samples are shown with the molecular weight standard in the top chromatograph and corresponding MW of peaks labeled in kDa. LdNH36-dg2 is present at retention time of 30 minutes corresponding to a MW of 132 kDa, demonstrating that LdNH36-dg2 is a tetramer in solution. The predicted structure of the molecule as a tetramer is shown in (B) with the mutated glutamines highlighted in red. (C) DLS results of the purified LdNH36-dg2 (SEC200 Pool) are presented as % intensity and demonstrate a similar MW (135 kDa). The polydispersity is 13.2%, indicating a monodisperse purified LdNH36-dg2.
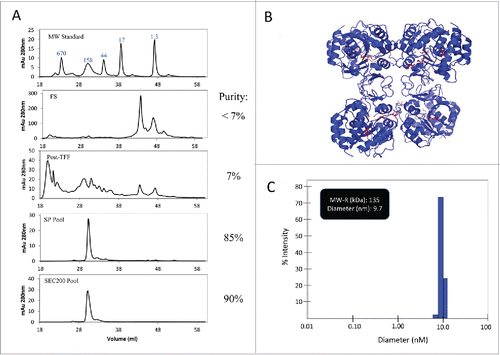
Figure 7. Scanning electron microscope (SEM) images of LdNH36-dg2- or CpG-loaded microparticles or empty microparticles. LdNH36-dg2 protein was encapsulated in poly(lactic-co-glycolic acid) (PLGA) microparticles using a water-oil-water double emulsion method, CpG oligonucleotide adjuvant was encapsulated using an oil-water emulsion method preceded by ion-pairing, and empty PLGA microparticles were prepared by an oil-water emulsion method.
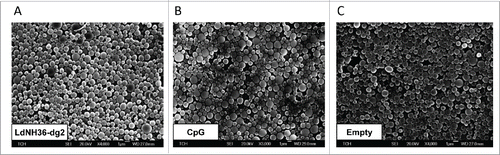
Figure 8. Serum antibody titers to (A) LdNH36-dg2 and (B) LdNH36-E-WT (non-polyhistidine tagged) for subtypes IgG1, IgG2a, and IgG2b in response to vaccination with microparticle-formulated LdNH36-dg2 protein and CpG or control formulations. BALB/c mice were vaccinated subcutaneously at weeks 0 and 3 with 40 μg of LdNH36-dg2 protein plus varying amounts of CpG adjuvant (10, 20, 40, or 80 μg) encapsulated in separate PLGA microparticles (MP). Vaccine controls included microparticle-LdNH36-dg2 and soluble LdNH36-dg2 + CpG. Mice were vaccinated in 2 cohorts, separated by 2 d (cohort 1, filled triangles; cohort 2, open squares). Week 5 serum was analyzed by ELISA for LdNH36-dg2- and LdNH36-E-WT-specific IgG1, IgG2a, and IgG2b responses. Negative control groups (microparticle-CpG, empty microparticle, and PBS) had antibody titers less than 103 and are not plotted. *p < 0.05, **p < 0.01, ***p < 0.001.
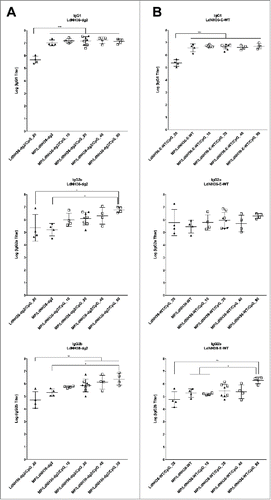
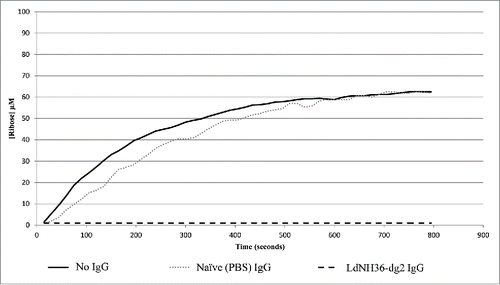
Figure 9. Antibody Inhibition of LdNH36-E-WT (non-polyhistidine tagged) Nucleoside Hydrolase Activity. LdNH36-E-WT alone shows hydrolysis of inosine to ribose that proceeds at a similar rate when purified IgG from sera of mice injected with PBS (naïve mice) is added to the reaction. Addition of purified IgG from sera of mice injected with LdNH36-dg2 completely inhibits the hydrolase activity.