Figures & data
Table 1. Demographic and clinical characteristics of COPD patients in the intradermal (ID) group (n = 75) and intramuscular (IM) group (n = 74).
Table 2. Hemagglutination inhibition (HI) antibody titers in the intradermal (ID) group (n = 75) compared with intramuscular (IM) group (n = 74) pre-vaccination and 4 weeks post-vaccination.
Table 3. Hemagglutination inhibition (HI) antibody titers in the intradermal (ID) group (n = 64) compared with intramuscular (IM) group (n = 62) pre-vaccination and 4 weeks post-vaccination in patients who had no previous vaccination containing influenza A(H1N1)pdm09 virus or no history of influenza A(H1N1)pdm09 infection.
Table 4. Hemagglutination inhibition (HI) antibody titers 4 weeks post-vaccination by baseline seronegative and seropositive status in the intradermal (ID) group (total n = 75) compared to intramuscular (IM) group (total n = 74).
Table 5. Side effects of influenza vaccination in the intradermal (ID) group (n = 67) and the intramuscular (IM) group (n = 67).
Figure 1. Numbers and types of acute respiratory illness (ARI) in the intradermal injection (ID, total events = 70) and intramuscular injection (IM, total events = 76) groups. The numbers of patients in each ARI type was not significantly different between the ID and IM groups.
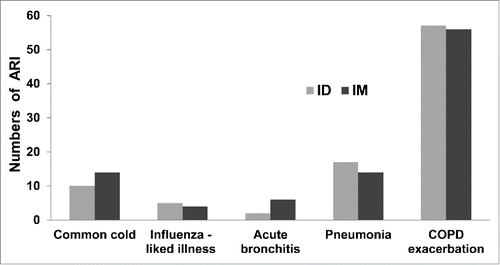
Table 6. Influenza-related acute respiratory illness diagnosed by hemagglutination inhibition (HI) titers or RT-PCR in the intradermal (ID) injection and intramuscular (IM) injection.
