Figures & data
Table 1. Results of CEA analysis broken down by different scenarios.
Figure 1. Incremental Cost-Effectiveness (ICE) ellipse scatterplots showing the distribution of values of incremental costs and incremental effectiveness resulting from the Monte Carlo simulation. Points below the threshold value indicate cost-effectiveness.
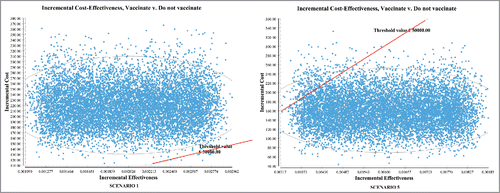
Figure 2. Cost-effectiveness acceptability curves (“vaccinate” and “do not vaccinate”). The figures show the probability of being cost-effective on varying the threshold value.
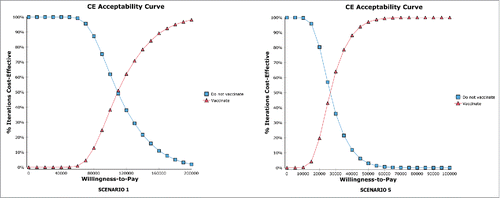
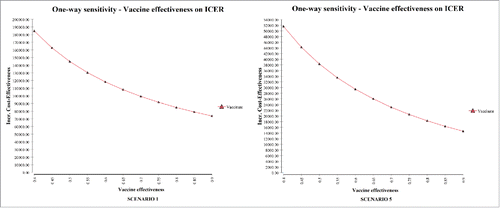
Figure 3. Impact of average annual incidence (per 100,000) of serogroup B invasive disease on the ICER (one-way sensitivity analysis). The probability of disease is per person.
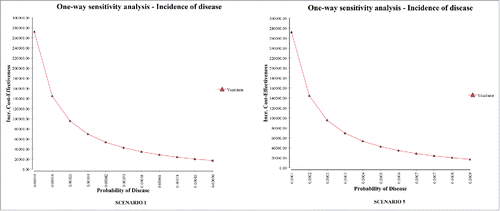
Figure 6. Simplified decisional tree: meningococcal serogroup B vaccination in infants.*Legend: amputation with substantial disability, anxiety, arthritis, depression, motor deficits, blindness, epilepsy or seizure, severe neurological disability, mental retardation (cognitive problems), hearing loss requiring cochlear implantation, moderate/severe bilateral hearing loss, moderate unilateral hearing loss, skin necrosis, scars, severe speech or communication problems, renal failure, chronic migraine.**Protection was assumed to begin after the second dose of the vaccine.
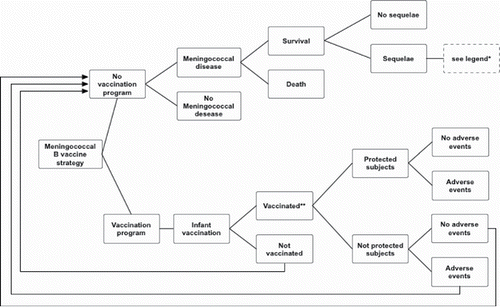
Table 2. Scenarios evaluated.
Table 3. Probability of sequelae.
Table 4. Health utilities of single sequelae.
Table 5. Acute phase of disease: costs (no discount rate) were measured in € at January 2013 values and were referred to 1 case.
Table 6. Meningococcal sequelae. Costs (no discount rate) were measured in € at January 2013 values.
Table 7. The social costs (no discount rate) of death were measured in € at January 2013 values.
Table 8. Costs associated with vaccination (€).