Figures & data
Figure 1. Subject disposition. aNumber of subjects excluded from the per-protocol MMRVAMP postdose 1 immunogenicity analyses: Measles (69); Mumps (80); Rubella (90); and Varicella (112). bNumber of subjects excluded from the per-protocol MMRV postdose 1 immunogenicity analyses: Measles (81); Mumps (92); Rubella (109); and Varicella (114). cOne subject did not receive Dose 2, but was followed for 180 d postdose 1 and discontinued due to an AE (onset was Day 5 postdose 1) during the extended safety follow-up but before study completion.
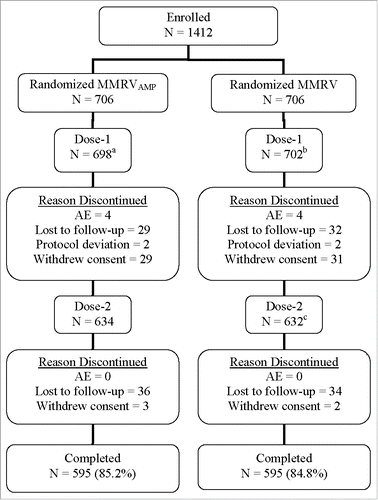
Table 1. Demographics.
Table 2. Summary of response rates and geometric mean concentrations (GMCs) after dose-1.
Table 3. Adverse experience (AE) summary (days 1 to 42 following each vaccination).
Table 4. Serious adverse experience (SAE) listing.
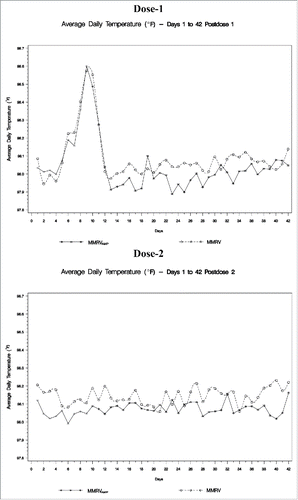
Table 5. Analysis of rates of fever by day range between vaccination groups - postdose 1 (all subjects as treated population).