Figures & data
Figure 1. Study design and patient flow. Participants were randomized 5:1:1 to receive IIV4, IIV3-1, or IIV3-2. IIV4 contained the 4 Northern Hemisphere 2013/2014 influenza strains recommended by the World Health Organization and the European Union: A/California/7/2009 (H1N1), A/Texas/50/2012 (H3N2), B/Brisbane/60/2008 (B Victoria lineage), and B/Massachusetts/02/20122012 (B Yamagata lineage). IIV3-1 contained both A strains and the B Victoria lineage strain. IIV3-2 contained both A strains and the B Yamagata lineage strain. All participants received one vaccination at day 0. Participants who had not received 2 doses of seasonal influenza vaccine during a previous season (i.e., unprimed participants) received a second dose of vaccine on day 28.
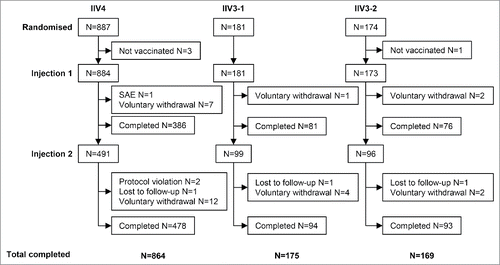
Table 1. Participant characteristics.
Table 2. Non-inferiority and superiority comparisons.
Table 3. HAI antibody responses.
Table 4. Safety summary: unsolicited AEs, solicited reactions, and SAEs after any injection.
Table 5. Solicited reactions with 7 d after any injection.
